Warnings
Ask a doctor before use if you have
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- keep dosing cup with product
- •
- mL = milliliter
- •
- this adult product is not intended for use in children under 12 years of age
|
age |
dose |
|
adults and children 12 years and over |
10 – 20 mL every 4 hours |
|
children under 12 years |
do not use |
Inactive ingredients
anhydrous citric acid, disodium edetate, FD&C red no. 40, flavor, potassium citrate, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xanthan gum.
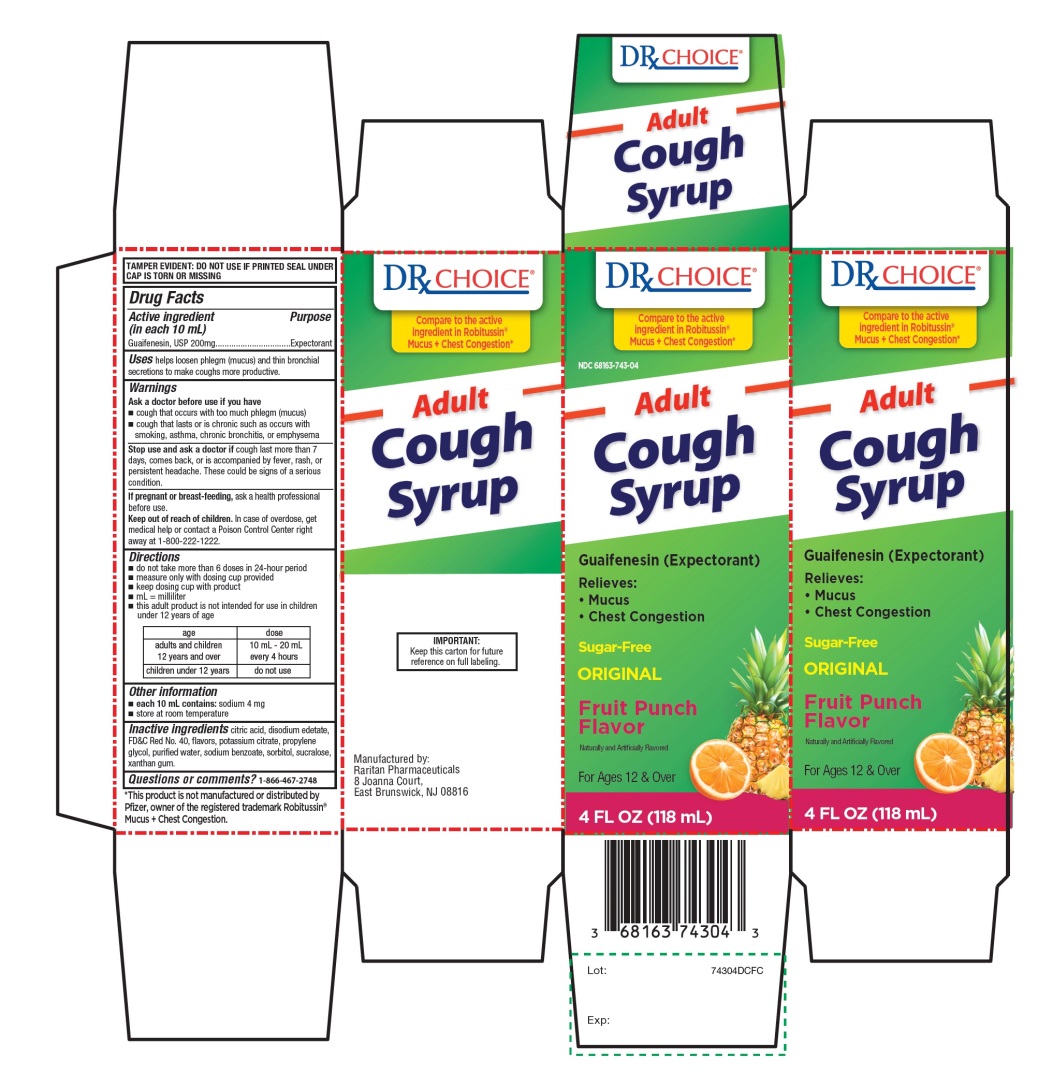
Principal Display Panel
DRx CHOICE®
NDC# 68163-743-04
Compare to the active ingredient in Robitussin® Mucus + Chest Congestion*
Adult Cough Syrup
Guaifenesin (Expectorant)
Relieves:
- •
- Mucus
- •
- Chest congestion
Sugar-Free
ORIGINAL
Fruit Punch Flavor
Naturally and Artificially Flavored
For Ages 12 & Over
4 FL OZ (118 mL)
|
IMPORTANT: Keep this carton for future reference on full labeling. |
*This product is not manufactured or distributed by Pfizer, owner of the registered trademark Robitussin® Mucus + Chest Congestion.
Manufactured by:
Raritan Pharmaceuticals
8 Joanna Court,
East Brunswick, NJ 08816