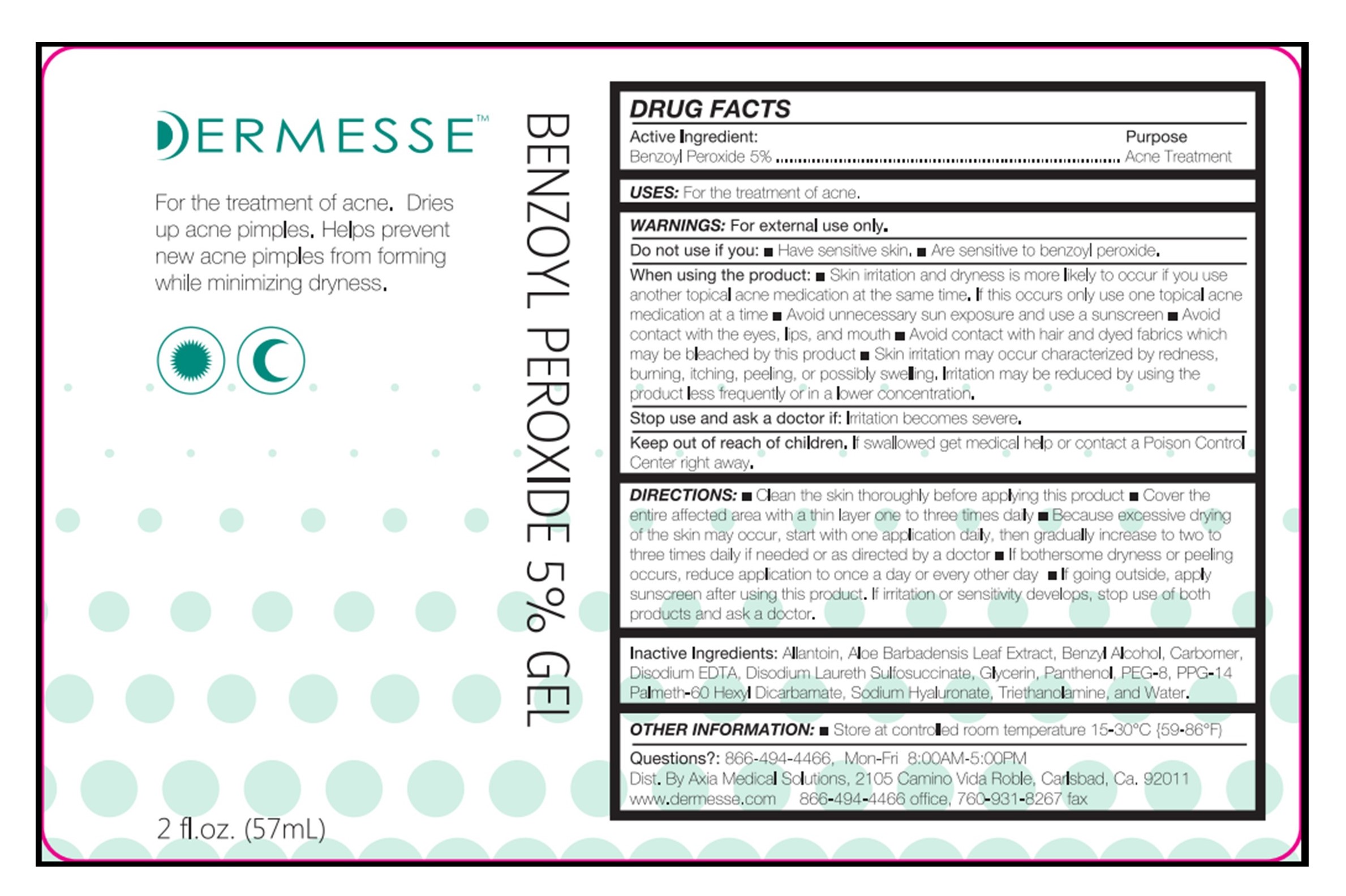
When using the product:
• Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If this occurs only use one topical acne medication at a time • Avoid unnecessary sun exposure and use a sunscreen • Avoid contact with the eyes, lips, and mouth • Avoid contact with hair and dyed fabrics which may be bleached by this product • Skin irritation may occur characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control Center right away.
DIRECTIONS:
• Clean the skin thoroughly before applying this product • Cover the entire affected area with a thin layer one to three times daily • Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a doctor • If bothersome dryness or peeling occurs, reduce application to once a day or every other day • If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
Inactive Ingredients:
Allantoin, Aloe Barbadensis Leaf Extract, Benzyl Alcohol, Carbomer, Disodium EDTA, Disodium Laureth Sulfosuccinate, Glycerin, Panthenol, PEG-8, PEG-14 Palmeth-60 Hexyl Dicarbamate, Sodium Hyaluronate, Triethanolamine, and Water.