Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
When using this product
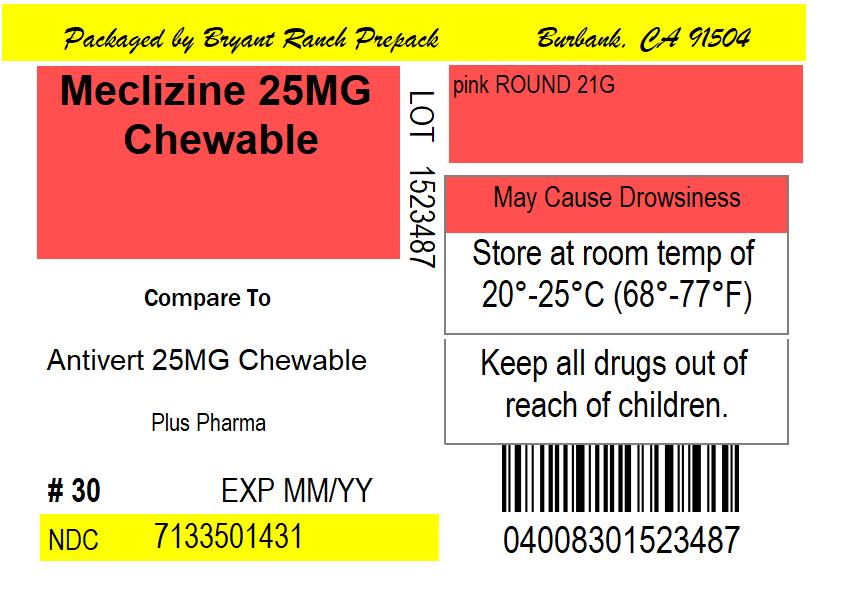
- may cause drowsiness
- alcohol, sedatives and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- use caution when driving a motor vehicle or operating machinery
Keep out of reach of children.
In case of overdose, get medical help or contact the poison control center immediately.
Directions
- dosage should be taken one hour before travel starts.
- adults and children 12 years of age and older: Chew 1-2 tablets once daily or as directed by a doctor
- children under 12 years: do not give this product to children under 12 years of age unless directed by a doctor
Other information
- phenylketonurics: contains phenylalanine 0.28 mg per tablet
- store at room temperature in a dry place
Inactive ingredients Aspartame, croscarmellose sodium, dextrose, FD&C red #40 lake, magnesium stearate, maltodextrin, microcrystalline cellulose, natural and artificial flavors, silicon dioxide, sodium sulfate, sugar, tricalcium phosphate.
Questions? If you have any questions or comments or to report an adverse event, please contact (800) 795-9775.
Distributed by: Plus Pharma, Commack, NY 11725
*Plus Pharma is not affiliated with the owner of the registered trademark Bonine®.
HOW SUPPLIED
NDC: 71335-0143-1: 30 Tablets in a BOTTLE
NDC: 71335-0143-2: 20 Tablets in a BOTTLE
NDC: 71335-0143-3: 25 Tablets in a BOTTLE
NDC: 71335-0143-4: 40 Tablets in a BOTTLE
NDC: 71335-0143-5: 60 Tablets in a BOTTLE
NDC: 71335-0143-6: 90 Tablets in a BOTTLE
NDC: 71335-0143-7: 8 Tablets in a BOTTLE
NDC: 71335-0143-8: 14 Tablets in a BOTTLE
NDC: 71335-0143-9: 10 Tablets in a BOTTLE
NDC: 71335-0143-0: 120 Tablets in a BOTTLE