Uses
- helps treat and prevent diaper rash
- protects chafed skin due to diaper rash and helps seal out wetness
Warnings
For external use only
Directions
- change wet and soiled diapers promptly
- cleanse the diaper area
- allow to dry
- apply cream liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
Inactive ingredients
Water, Mineral Oil, Petrolatum, Beeswax, Dimethicone, Sorbitan Sesquioleate, Microcrystalline Wax, PEG-30 Dipolyhydroxystearate, Aloe Barbadensis Leaf Extract, Glycerin, Tropolone, Tocopheryl Acetate, 1,2-Hexanediol, Caprylyl Glycol, Magnesium Sulfate, Potassium Hydroxide, Phenoxyethanol
Questions?
Call toll-free 800-720-3843 or 215-273-8755 (collect)
For more information on preventing and treating diaper rash, visit www.desitin.com
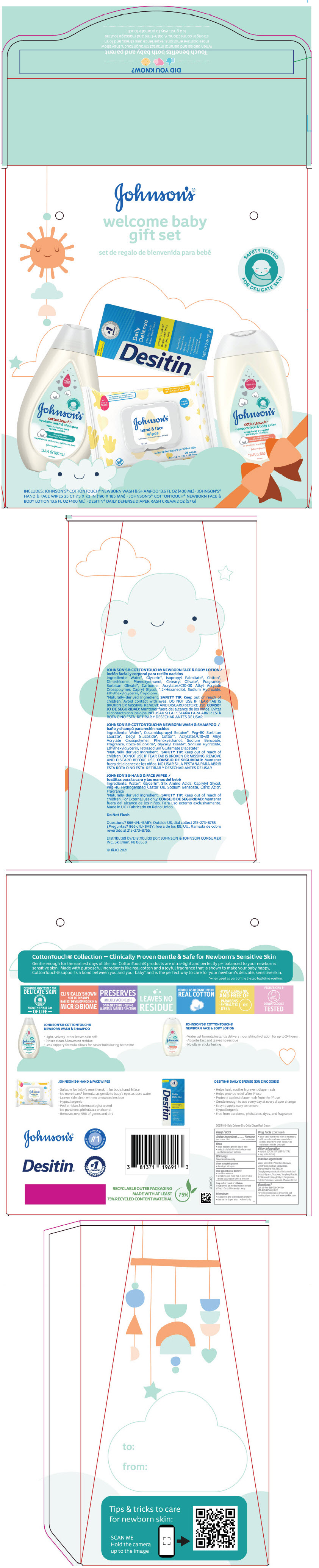
PRINCIPAL DISPLAY PANEL - Kit Package
JOHNSON'S
®
welcome baby
gift set
set de regalo de bienvenida para bebé
SAFETY TESTED
FOR DELICATE SKIN
INCLUDES: JOHNSON'S
® COTTONTOUCH® NEWBORN WASH & SHAMPOO 13.6 FL OZ (400 mL) ● JOHNSON'S
®
HAND & FACE WIPES 25 CT 7.5 × 7.3 IN (190 × 185 MM) ● JOHNSON'S
® COTTONTOUCH
® NEWBORN FACE &
BODY LOTION 13.6 FL OZ (400 mL) ● DESITIN
® DAILY DEFENSE DIAPER RASH CREAM 2 OZ (57 G)