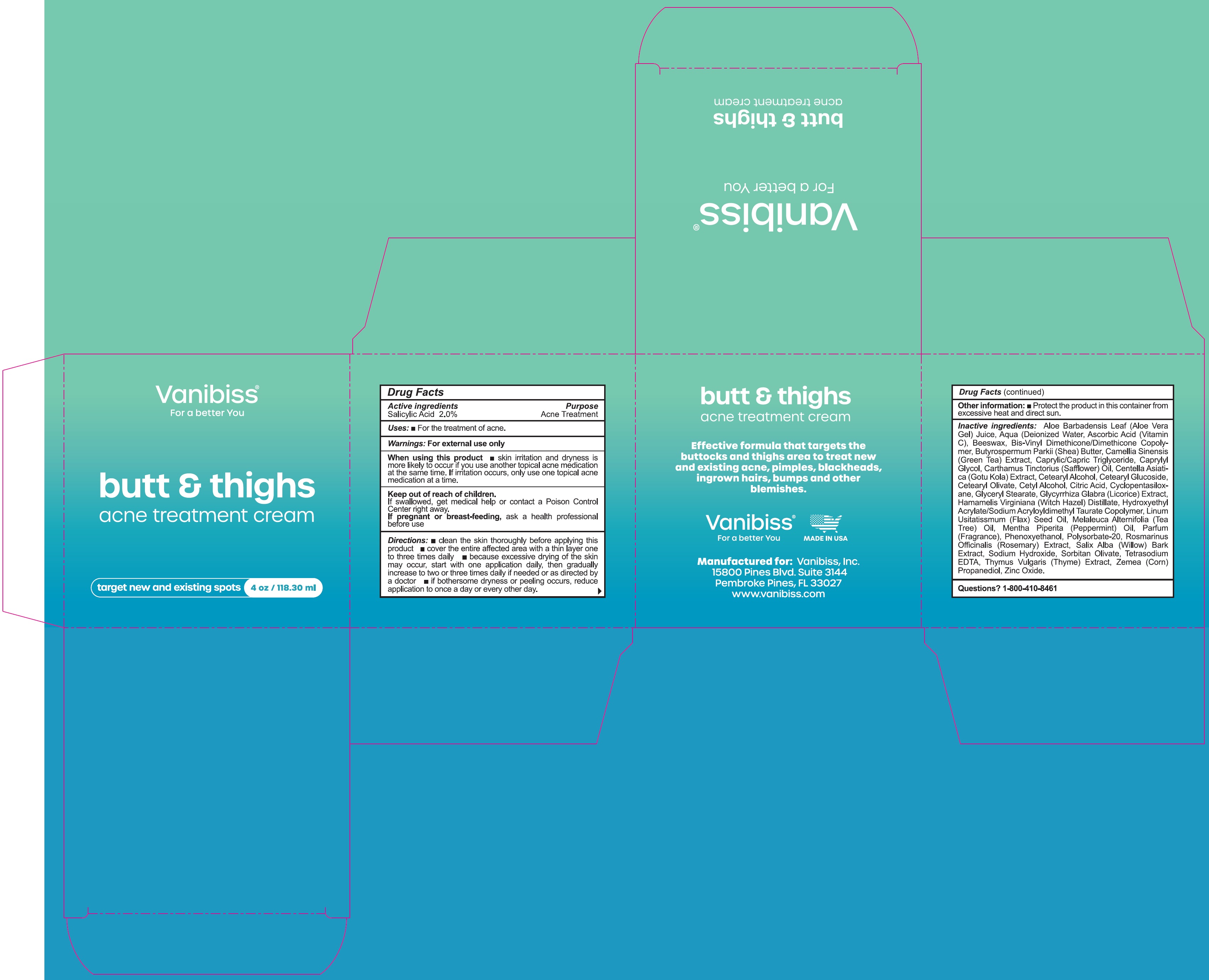
Warnings:
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Directions:
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily, if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Ascorbic Acid (Vitamin C), Beeswax, Bis-Vinyl Dimethicone/Dimethicone Copolymer, Butyrospermum Parkii (Shea) Butter, Camellia Sinensis (Green Tea) Extract, Caprylic/Capric Triglyceride, Caprylyl Glycol, Carthamus Tinctorius (Safflower) Oil, Centella Asiatica (Gotu Kola) Extarct, Cetearyl Alcohol, Cetearyl Glucoside, Cetearyl Olivate, Cetyl Alcohol, Citric Acid, Cyclopentasiloxane, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Extract, Hamamelis Virginiana (Witch Hazel) Distillate, Hydroxyethyl Acrylate/Sodium (Flax) Seed Oil, Mentha Piperita (Peppermint) Oil, Parfum (Fragrance), Phenoxyethanol, Polysorbate-20, Rosmarinus Officinalis (Rosemary) Extarct, Salix Alba (Willow) Bark Extarct, Sodium Hydroxide, Sorbitan Olivate, Tetrasodium EDTA, Thymus Vulgaris (Thyme) Extarct, Zemea (Corn) propanediol, Zinc Oxide.