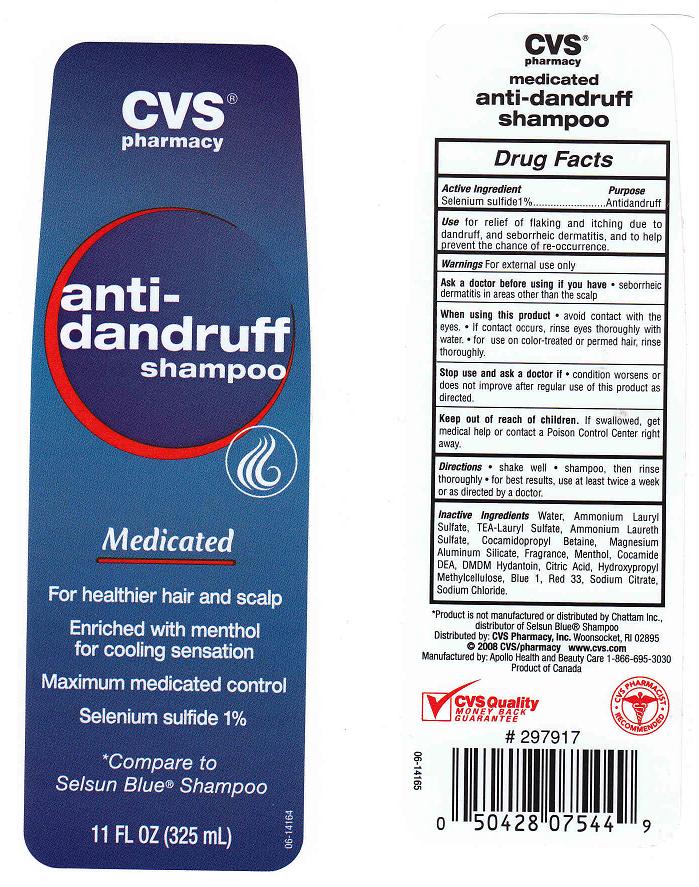
ANTIDANDRUFF MEDICATED- selenium sulfide shampoo
CVS PHARMACY INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
SELENIUM SULFIDE 1%
WARNINGS
ASK A DOCTOR BEFORE USING IF YOU HAVE
SEBORRHEIC DERMATITIS IN AREAS OTHER THAN THE SCALP
WHEN USING THIS PRODUCT
- AVOID CONTACT WITH THE EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER. FOR USE ON COLOR-TREATED OR PERMED HAIR, RINSE THOROUGHLY.
STOP USE AND ASK A DOCTOR IF
- CONDITION WORSENS OR DOES NOT IMPROVE AFTER REGULAR USE OF THIS PRODUCT AS DIRECTED.
KEEP OUT OF REACH OF CHILDREN
- IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
USE
FOR RELIEF OF FLAKING AND ITCHING DUE TO DANDRUFF, AND SEBORRHEIC DERMATITIS, AND TO HELP PREVENT THE CHANCE OF RE-OCCURRENCE.
DIRECTIONS
- SHAKE WELL, SHAMPOO, THEN RINSE THROUGHLY.
- FOR BEST RESULTS, USE AT LEAST TWICE A WEEK OR AS DIRECTED BY A DOCTOR.
INACTIVE INGREDIENTS
WATER, AMMONIUM LAURYL SULFATE, TEA-LAURYL SULFATE, AMMONIUM LAURETH SULFATE, COCAMIDOPROPYL BETAINE, MAGNESIUM ALUMINUM SILICATE, FRAGRANCE, MENTHOL, COCAMIDE DEA, DMDM HYDANTOIN, CITRIC ACID, HYDROXYPROPYL METHYLCELLULOSE, SODIUM CITRATE, SODIUM CHLORIDE, BLUE 1 (CI 42090), RED 33 (CI 17200)
PACKAGE FRONT AND BACK LABELS
FRONT AND BACK LABELS: cvs11.jpg