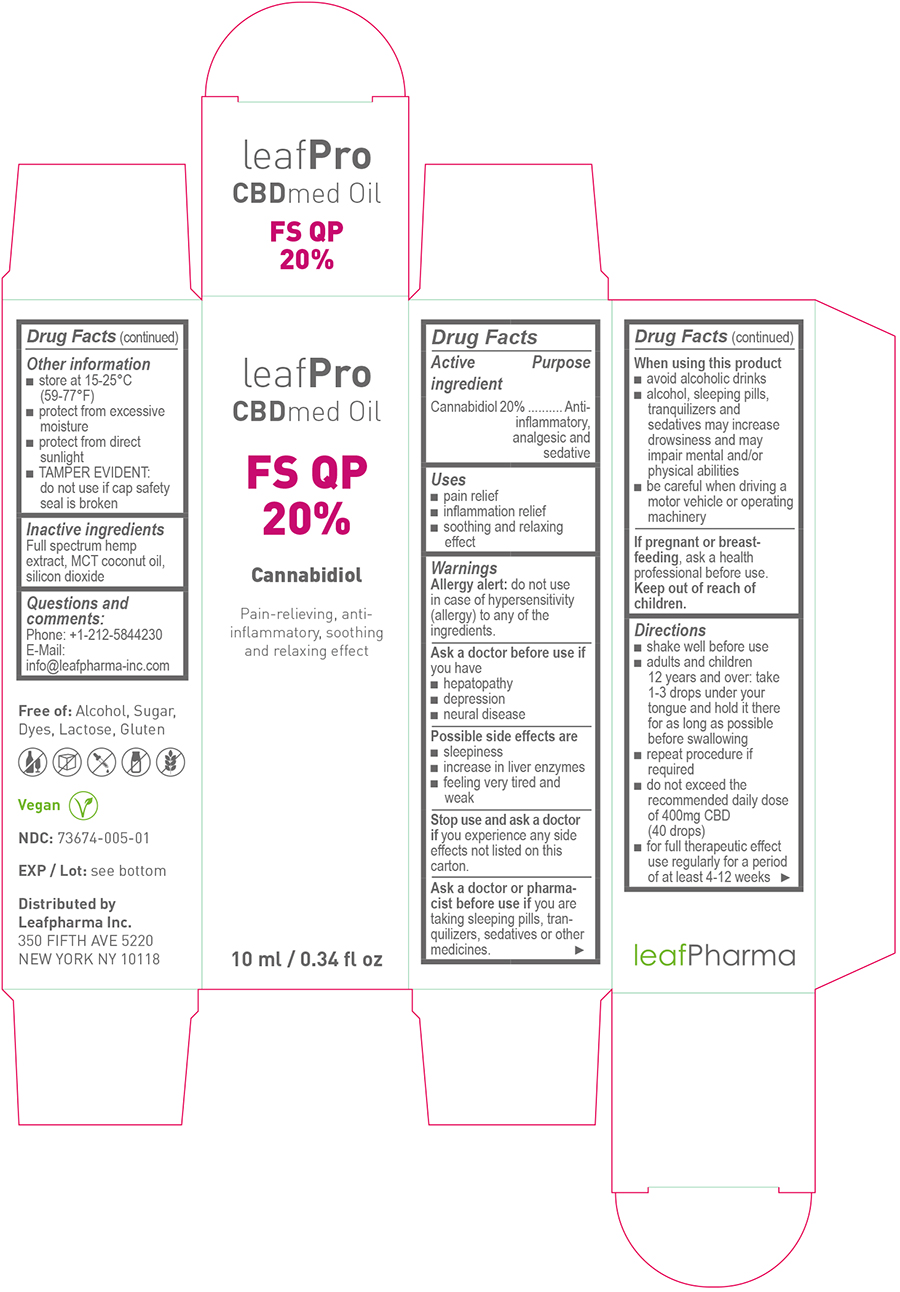
Active Ingredient
Cannabidiol 20%
Purpose
Anti-inflammatory, analgesic and sedative
Uses
- pain relief
- inflammation relief
- soothing and relaxing effect
Warnings
Allergy alert:
do not use in case of hypersensitivity (allergy) to any of the ingredients.
Ask a doctor before use if
you have
- hepatopathy
- depression
- neural disease
Possible side effects are
- sleepiness
- increase in liver enzymes
- feeling very tired and weak
Stop use and ask a doctor if
you experience any side effects not listed on this carton.
Ask a doctor or pharmacist before use if
you are taking sleeping pills, tranquilizers, sedatives or other medicines.
When using this product
- avoid alcoholic drinks
- alcohol, sleeping pills, tranquilizers and sedatives may increase drowsiness and may impair mental and/or physical abilities
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
Directions
- shake well before use
- adults and children 12 years and over: take 1 – 3 drops under your tongue and hold it there for as long as possible before swallowing
- repeat procedure if required
- do not exceed the recommended daily dose of 400mg CBD (40 drops)
- for full therapeutic effect use regularly for a period of at least 4-12 weeks
Other information
- store at 15-25°C (59-77°F)
- protect from excessive moisture
- protect from direct sunlight
- TAMPER EVIDENT: do not use if cap safety seal is broken
Inactive ingredients:
Full spectrum hemp extract, MCT coconut oil, silicon dioxide
Questions and comments:
Phone: +1-212-5844230
E-Mail: info@leafpharma-inc.com
Principal Display Panel - 10ml
leaf
Pro
CBDmed Oil
FS QP
20%
Cannabidiol
Pain-relieving, anti-inflammatory, soothing and relaxing effect
10ml / 0.34 fl oz