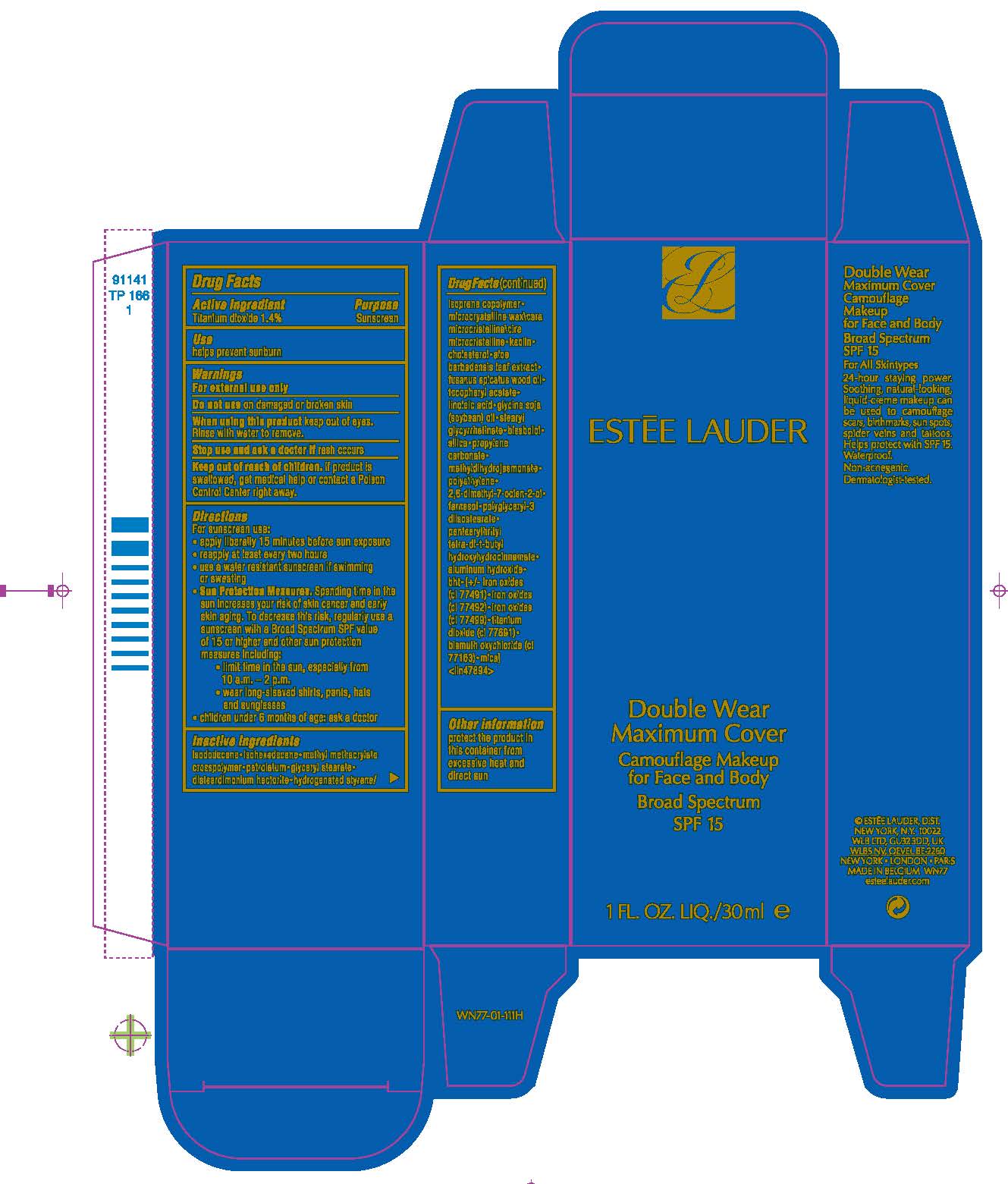
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
Inactive ingredients
isododecane•isohexadecane•methyl methacrylate crosspolymer•petrolatum•glyceryl stearate•disteardimonium hectorite•hydrogenated styrene/isoprene copolymer•microcrystalline wax\cera microcristallina\cire microcristalline•kaolin•cholesterol•aloe barbadensis leaf extract•fusanus spicatus wood oil•tocopheryl acetate•linoleic acid•glycine soja (soybean) oil•stearyl glycyrrhetinate•bisabolol•silica•propylene carbonate•methyldihydrojasmonate•polyethylene•2,6-dimethyl-7-octen-2-ol•polyglyceryl-3 diisostearate•pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate• alumina•phenethyl alcohol•[+/- iron oxides (ci 77491)•iron oxides (ci 77492)•iron oxides (ci 77499)•titanium dioxide (ci 77891)•mica•bismuth oxychloride (ci 77163)] <iln40794>