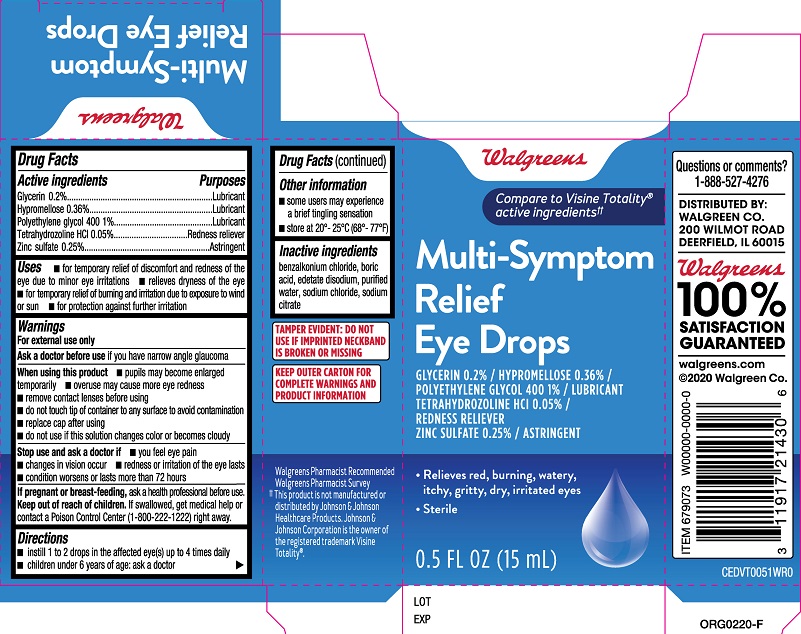
Active ingredients
Glycerin ....0.2%
Hypromellose.....0.36%
Polyethylene glycol 400.....1%
Tetrahydrozoline HCl.....0.05%
Zinc sulfate 0.25%
Purposes
Glycerin ....Lubricant
Hypromellose.....Lubricant
Polyethylene glycol 400.....Lubricant
Tetrahydrozoline HCl.....Redness reliever
Zinc sulfate.....Astringent
Uses
- for temporary relief of discomfort and redness of the eye due to minor eye irritations
- relieves dryness of the eye
- for temporary relief of burning and irritation due to exposure to wind or sun
- for protection against further irritation
Uses
- for temporary relief of discomfort and redness of the eye due to minor eye irritations
- relieves dryness of the eye
- for temporary relief of burning and irritation due to exposure to wind or sun
- for protection against further irritation
Warnings
For external use only
When using this product
- pupils may become enlarged temporarily
- overuse may cause more eye redness
- remove contact lenses before using
- do not touch tip of container to any surface to avoid contamination
- replace cap after using
- do not use if this solution changes color or becomes cloudy
Directions
- instill 1 to 2 drops in the affected eye(s) up to 4 times daily
- children under 6 years of age: ask a doctor