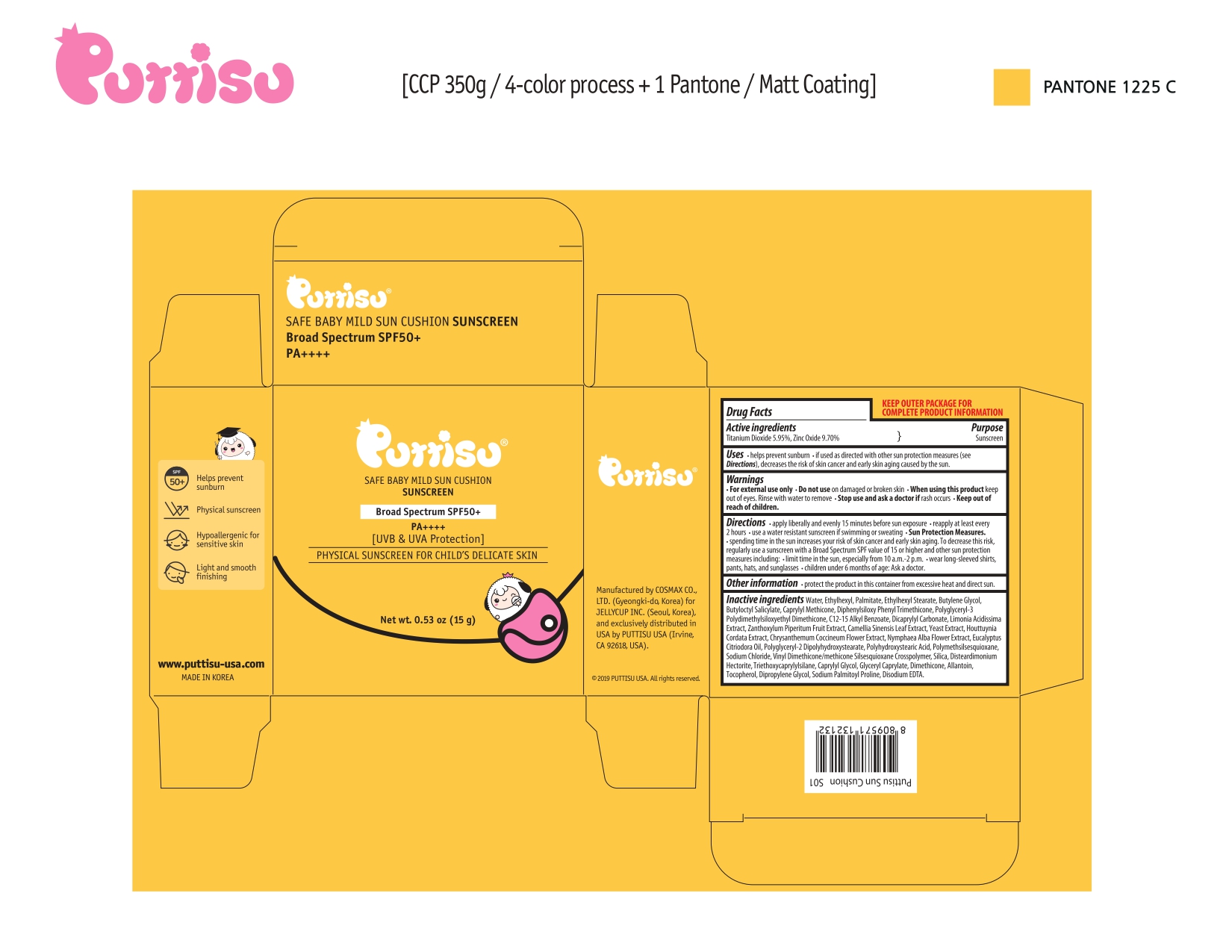
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Applying liberally an evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- Sun Protection Measures
- spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or high and other sun protection measures including:
- limit time in the sun, especially from 10 am-2 pm
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months age: Ask a doctor
WATER
ETHYLHEXYL PALMITATE
ETHYLHEXYL STEARATE
BUTYLENE GLYCOL
BUTYLOCTYL SALICYLATE
CAPRYLYL TRISILOXANE
DIPHENYLSILOXY PHENYL TRIMETHICONE
POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S)
ALKYL (C12-15) BENZOATE
DICAPRYLYL CARBONATE
LIMONIA ACIDISSIMA BARK
ZANTHOXYLUM PIPERITUM FRUIT PULP
GREEN TEA LEAF
YEAST, UNSPECIFIED
HOUTTUYNIA CORDATA TOP
TANACETUM COCCINEUM FLOWER
NYMPHAEA ALBA FLOWER
CORYMBIA CITRIODORA LEAF OIL
POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE
POLYHYDROXYSTEARIC ACID STEARATE
POLYMETHYLSILSESQUIOXANE (11 MICRONS)
SODIUM CHLORIDE
VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER
SILICON DIOXIDE
DISTEARDIMONIUM HECTORITE
TRIETHOXYCAPRYLYLSILANE
CAPRYLYL GLYCOL
GLYCERYL CAPRYLATE
DIMETHICONE
ALLANTOIN
TOCOPHEROL
DIPROPYLENE GLYCOL
SODIUM PALMITOYL PROLINE
DISODIUM EDTA-COPPER