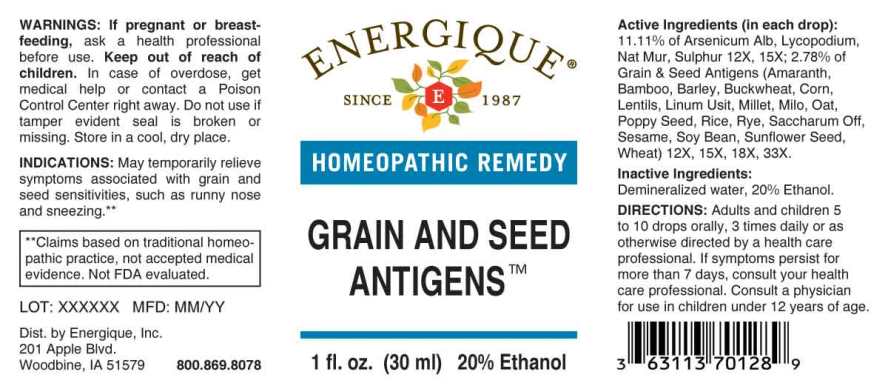
ACTIVE INGREDIENTS:
(in each drop): 11.11% of Arsenicum Album 12X, 15X, Lycopodium Clavatum 12X, 15X, Natrum Muriaticum 12X, 15X, Sulphur 12X, 15X; 2.78% of Bamboo 12X, 15X, 18X, 33X, Barley 12X, 15X, 18X, 33X, Corn 12X, 15X, 18X, 33X, Cotton Seed 12X, 15X, 18X, 33X, Flax Seed (Linum Usitatissimum) 12X, 15X, 18X, 33X, Millet 12X, 15X, 18X, 33X, Milo 12X, 15X, 18X, 33X, Oat 12X, 15X, 18X, 33X, Poppy Seed 12X, 15X, 18X, 33X, Rice 12X, 15X, 18X, 33X, Rye 12X, 15X, 18X, 33X, Safflower 12X, 15X, 18X, 33X, Sesame 12X, 15X, 18X, 33X, Sorghum 12X, 15X, 18X, 33X, Soy Bean 12X, 15X, 18X, 33X, Sugarcane 12X, 15X, 18X, 33X, Sunflower (Helianthus Annuus) 12X, 15X, 18X, 33X, Wheat 12X, 15X, 18X, 33X.
INDICATIONS:
May temporarily relieve symptoms associated with grains and seed allergies such as runny nose and sneezing.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist for more than 7 days, consult your health care professional. Consult a physician for use in children under 12 years of age.