FULL PRESCRIBING INFORMATION
WARNING: SERIOUS INFECTIONS and MALIGNANCIES
SERIOUS INFECTIONS
Patients treated with Enbrel are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1) and Adverse Reactions (6)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Enbrel should be discontinued if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Test patients for latent tuberculosis before Enbrel use and during therapy. Initiate treatment for latent infection prior to Enbrel use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral, and other infections due to opportunistic pathogens, including Legionella and Listeria.
The risks and benefits of treatment with Enbrel should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Monitor patients closely for the development of signs and symptoms of infection during and after treatment with Enbrel, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
1 INDICATIONS AND USAGE
1.1 Rheumatoid Arthritis
Enbrel is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis (RA). Enbrel can be initiated in combination with methotrexate (MTX) or used alone.
1.2 Polyarticular Juvenile Idiopathic Arthritis
Enbrel is indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA) in patients 2 years of age and older.
1.3 Psoriatic Arthritis
Enbrel is indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in adult patients with psoriatic arthritis (PsA). Enbrel can be used with or without methotrexate.
1.4 Ankylosing Spondylitis
Enbrel is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis (AS).
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Procedures Prior to Treatment Initiation
Perform the following evaluations and procedures prior to initiating treatment with Enbrel:
- Prior to initiating Enbrel and periodically during therapy, evaluate patients for active tuberculosis and test for latent infection [see Warnings and Precautions (5.1)].
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with Enbrel [see Warnings and Precautions (5.8)].
2.2 Important Administration Instructions
Administration of one 50 mg Enbrel single-dose prefilled syringe, one single-dose prefilled Enbrel SureClick autoinjector, or one Enbrel Mini single-dose prefilled cartridge (for use with the AutoTouch reusable autoinjector only), provides a dose equivalent to two 25 mg Enbrel single-dose prefilled syringes, two 25 mg single-dose vials, or two multiple-dose vials of lyophilized Enbrel, when multiple-dose vials are reconstituted and administered as recommended.
2.3 Recommended Dosage in Adult Patients with Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis, and Plaque Psoriasis
Enbrel is administered by subcutaneous injection (Table 1).
| Patient Population | Recommended Dosage |
|---|---|
| Adult RA, AS, and PsA | 50 mg weekly |
| Adult PsO | Starting Dose: 50 mg twice weekly for 3 months Maintenance Dose: 50 mg once weekly |
See the Enbrel (etanercept) "Instructions for Use" insert for detailed information on injection site selection and dose administration [see Dosage and Administration (2.3) and Patient Counseling Information (17)].
Adult Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients
Methotrexate, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with Enbrel.
Based on a study of 50 mg Enbrel twice weekly in patients with RA that suggested higher incidence of adverse reactions but similar American College of Rheumatology (ACR) response rates, doses higher than 50 mg per week are not recommended.
Adult Plaque Psoriasis Patients
In addition to the 50 mg twice weekly recommended starting dose, starting doses of 25 mg or 50 mg per week were shown to be efficacious. The proportion of responders was related to Enbrel dosage [see Clinical Studies (14.5)].
2.4 Recommended Dosage for Pediatric Patients with Polyarticular Juvenile Idiopathic Arthritis, Plaque Psoriasis, and Juvenile Psoriatic Arthritis
The recommended weight-based dosage for pediatric patients is administered by subcutaneous injection (Table 2).
| Body Weight | Recommended Dosage |
|---|---|
| 63 kg (138 pounds) or more | 50 mg weekly |
| Less than 63 kg (138 pounds) | 0.8 mg/kg weekly |
To achieve pediatric doses other than 25 mg or 50 mg, use Enbrel solution in a single-dose vial or reconstituted lyophilized powder in a multiple-dose vial.
Dosages of Enbrel higher than those described in Table 2 have not been studied in pediatric patients.
In pJIA patients, glucocorticoids, NSAIDs, or analgesics may be continued during treatment with Enbrel.
2.5 Preparation Instructions for Enbrel
Enbrel is intended for use under the guidance and supervision of a physician. Patients may self-inject when deemed appropriate and if they receive medical follow-up, as necessary. Patients should not self-administer until they receive proper training in how to prepare and administer the correct dose. Administer injections subcutaneously in the thigh, abdomen or outer area of the upper arm.
The Enbrel devices are not made with natural rubber latex.
The Enbrel (etanercept) "Instructions for Use" insert for each presentation contains more detailed instructions on injection site selection and the preparation of Enbrel.
Preparation of Enbrel Single-dose Prefilled Syringe
For a more comfortable injection, leave Enbrel prefilled syringes at room temperature for about 15 to 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose prefilled syringe, check to see if the amount of liquid in the prefilled syringe falls between the two purple fill level indicator lines on the syringe. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE.
Preparation of Enbrel Single-dose Prefilled SureClick Autoinjector
Leave the autoinjector at room temperature for at least 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
Preparation of Enbrel Single-dose Vial
For a more comfortable injection, leave Enbrel vial(s) at room temperature for at least 30 minutes before injecting. DO NOT remove the vial cap while allowing the vial to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose vial, administer the correct dose of solution using the following recommended materials:
- A 1 mL Luer-Lock syringe.
- A withdrawal needle with Luer-Lock connection, sterile, 22-gauge, length 1 ½ inch.
- An injection needle with Luer-Lock connection, sterile, 27-gauge, length ½ inch.
Two vials may be required to administer the total prescribed dose. Use the same syringe for each vial. The vial does not contain preservatives; therefore, discard unused portions.
Preparation of Enbrel Lyophilized Powder in a Multiple-dose Vial
Enbrel lyophilized powder should be reconstituted aseptically with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), giving a solution of 1 mL containing 25 mg of Enbrel.
A vial adapter is supplied for use when reconstituting the lyophilized powder. However, the vial adapter should not be used if multiple doses are going to be withdrawn from the vial. If the vial will be used for multiple doses, a 25-gauge needle should be used for reconstituting and withdrawing Enbrel, and the supplied "Mixing Date:" sticker should be attached to the vial and the date of reconstitution entered. Reconstituted solution must be refrigerated at 36°F to 46°F (2°C to 8°C) and used within 14 days. Discard reconstituted solution after 14 days because product stability and sterility cannot be assured after 14 days. DO NOT store reconstituted Enbrel solution at room temperature.
For a more comfortable injection, leave the Enbrel dose tray at room temperature for about 15 to 30 minutes before injecting.
If using the vial adapter, twist the vial adapter onto the diluent syringe. Then, place the vial adapter over the Enbrel vial and insert the vial adapter into the vial stopper. Push down on the plunger to inject the diluent into the Enbrel vial. If using a 25-gauge needle to reconstitute and withdraw Enbrel, the diluent should be injected very slowly into the Enbrel vial. It is normal for some foaming to occur. Keeping the diluent syringe in place, gently swirl the contents of the Enbrel vial during dissolution. To avoid excessive foaming, do not shake or vigorously agitate.
Generally, dissolution of Enbrel takes less than 10 minutes. Do not use the solution if discolored or cloudy, or if particulate matter remains.
Withdraw the correct dose of reconstituted solution into the syringe. Some foam or bubbles may remain in the vial. Remove the syringe from the vial adapter or remove the 25-gauge needle from the syringe. Attach a 27-gauge needle to inject Enbrel.
The contents of one vial of Enbrel solution should not be mixed with, or transferred into, the contents of another vial of Enbrel. No other medications should be added to solutions containing Enbrel, and do not reconstitute Enbrel with other diluents. Do not filter reconstituted solution during preparation or administration.
Preparation of Enbrel Mini® single-dose prefilled cartridge using the AutoTouch® reusable autoinjector
Leave Enbrel Mini single-dose prefilled cartridge at room temperature for at least 30 minutes before injecting. DO NOT remove the purple cap while allowing the cartridge to reach room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
To use AutoTouch reusable autoinjector, open the door by pushing the door button and inserting Enbrel Mini single-dose prefilled cartridge into AutoTouch. When inserted correctly, Enbrel Mini single-dose prefilled cartridge will slide freely and completely into the door. Close the door and AutoTouch reusable autoinjector is ready for injection.
3 DOSAGE FORMS AND STRENGTHS
- Injection: 25 mg/0.5 mL and 50 mg/mL clear, colorless solution in a single-dose prefilled syringe
- Injection: 50 mg/mL clear, colorless solution in a single-dose prefilled SureClick autoinjector
- Injection: 25 mg/0.5 mL clear, colorless solution in a single-dose vial
- For Injection: 25 mg lyophilized powder in a multiple-dose vial for reconstitution
- Injection: 50 mg/mL clear, colorless solution in Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector only
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
Patients treated with Enbrel are at increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, parasitic, or other opportunistic pathogens including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis, and tuberculosis have been reported with TNF-blockers. Patients have frequently presented with disseminated rather than localized disease.
Treatment with Enbrel should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants (such as corticosteroids or methotrexate), may be at greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- With chronic or recurrent infection;
- Who have been exposed to tuberculosis;
- With a history of an opportunistic infection;
- Who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis; or
- With underlying conditions that may predispose them to infection, such as advanced or poorly controlled diabetes [see Adverse Reactions (6.1)].
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with Enbrel.
Enbrel should be discontinued if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with Enbrel should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
Tuberculosis
Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving Enbrel, including patients who have previously received treatment for latent or active tuberculosis. Data from clinical trials and preclinical studies suggest that the risk of reactivation of latent tuberculosis infection is lower with Enbrel than with TNF-blocking monoclonal antibodies. Nonetheless, postmarketing cases of tuberculosis reactivation have been reported for TNF-blockers, including Enbrel. Tuberculosis has developed in patients who tested negative for latent tuberculosis prior to initiation of therapy. Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating Enbrel and periodically during therapy. Tests for latent tuberculosis infection may be falsely negative while on therapy with Enbrel.
Treatment of latent tuberculosis infection prior to therapy with TNF-blocking agents has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating Enbrel, even for patients previously vaccinated with Bacillus Calmette-Guerin (BCG).
Anti-tuberculosis therapy should also be considered prior to initiation of Enbrel in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
Tuberculosis should be strongly considered in patients who develop a new infection during Enbrel treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
Invasive Fungal Infections
Cases of serious and sometimes fatal fungal infections, including histoplasmosis, have been reported with TNF-blockers, including Enbrel. For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric anti-fungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric anti-fungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and the risks of anti-fungal therapy. In 38 Enbrel clinical trials and 4 cohort studies in all approved indications representing 27,169 patient-years of exposure (17,696 patients) from the United States and Canada, no histoplasmosis infections were reported among patients treated with Enbrel.
5.2 Neurologic Reactions
Treatment with TNF-blocking agents, including Enbrel, has been associated with rare (< 0.1%) cases of new onset or exacerbation of central nervous system demyelinating disorders, some presenting with mental status changes and some associated with permanent disability, and with peripheral nervous system demyelinating disorders. Cases of transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barre syndromes, other peripheral demyelinating neuropathies, and new onset or exacerbation of seizure disorders have been reported in postmarketing experience with Enbrel therapy. Prescribers should exercise caution in considering the use of Enbrel in patients with preexisting or recent-onset central or peripheral nervous system demyelinating disorders [see Postmarketing Experience (6.3)].
5.3 Malignancies
Lymphomas
In the controlled portions of clinical trials of TNF-blocking agents, more cases of lymphoma have been observed among patients receiving a TNF-blocker compared to control patients. During the controlled portions of Enbrel trials in adult patients with RA, AS, and PsA, 2 lymphomas were observed among 3306 Enbrel-treated patients versus 0 among 1521 control patients (duration of controlled treatment ranged from 3 to 36 months).
Among 6543 adult rheumatology (RA, PsA, AS) patients treated with Enbrel in controlled and uncontrolled portions of clinical trials, representing approximately 12,845 patient-years of therapy, the observed rate of lymphoma was 0.10 cases per 100 patient-years. This was 3-fold higher than the rate of lymphoma expected in the general U.S. population based on the Surveillance, Epidemiology, and End Results (SEER) Database. An increased rate of lymphoma up to several-fold has been reported in the RA patient population, and may be further increased in patients with more severe disease activity.
Among 4410 adult PsO patients treated with Enbrel in clinical trials up to 36 months, representing approximately 4278 patient-years of therapy, the observed rate of lymphoma was 0.05 cases per 100 patient-years, which is comparable to the rate in the general population. No cases were observed in Enbrel- or placebo-treated patients during the controlled portions of these trials.
Leukemia
Cases of acute and chronic leukemia have been reported in association with postmarketing TNF-blocker use in rheumatoid arthritis and other indications. Even in the absence of TNF-blocker therapy, patients with rheumatoid arthritis may be at higher risk (approximately 2-fold) than the general population for the development of leukemia.
During the controlled portions of Enbrel trials, 2 cases of leukemia were observed among 5445 (0.06 cases per 100 patient-years) Enbrel-treated patients versus 0 among 2890 (0%) control patients (duration of controlled treatment ranged from 3 to 48 months).
Among 15,401 patients treated with Enbrel in controlled and open portions of clinical trials representing approximately 23,325 patient-years of therapy, the observed rate of leukemia was 0.03 cases per 100 patient-years.
Other Malignancies
Information is available from 10,953 adult patients with 17,123 patient-years and 696 pediatric patients with 1282 patient-years of experience across 45 Enbrel clinical studies.
For malignancies other than lymphoma and non-melanoma skin cancer, there was no difference in exposure-adjusted rates between the Enbrel and control arms in the controlled portions of clinical studies for all indications. Analysis of the malignancy rate in combined controlled and uncontrolled portions of studies has demonstrated that types and rates are similar to what is expected in the general U.S. population based on the SEER database and suggests no increase in rates over time. Whether treatment with Enbrel might influence the development and course of malignancies in adults is unknown.
Melanoma and Non-Melanoma Skin Cancer (NMSC)
Melanoma and non-melanoma skin cancer has been reported in patients treated with TNF antagonists including etanercept.
Among 15,401 patients treated with Enbrel in controlled and open portions of clinical trials representing approximately 23,325 patient-years of therapy, the observed rate of melanoma was 0.043 cases per 100 patient-years.
Among 3306 adult rheumatology (RA, PsA, AS) patients treated with Enbrel in controlled clinical trials representing approximately 2669 patient-years of therapy, the observed rate of NMSC was 0.41 cases per 100 patient-years versus 0.37 cases per 100 patient-years among 1521 control-treated patients representing 1077 patient-years. Among 1245 adult PsO patients treated with Enbrel in controlled clinical trials, representing approximately 283 patient-years of therapy, the observed rate of NMSC was 3.54 cases per 100 patient-years versus 1.28 cases per 100 patient-years among 720 control-treated patients representing 156 patient-years.
Postmarketing cases of Merkel cell carcinoma have been reported very infrequently in patients treated with Enbrel.
Periodic skin examinations should be considered for all patients at increased risk for skin cancer.
Pediatric Patients
Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy at ≤ 18 years of age), including Enbrel. Approximately half the cases were lymphomas, including Hodgkin's and non-Hodgkin's lymphoma. The other cases represented a variety of different malignancies and included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months of therapy (range 1 to 84 months). Most of the patients were receiving concomitant immunosuppressants. These cases were reported postmarketing and are derived from a variety of sources, including registries and spontaneous postmarketing reports.
In clinical trials of 1140 pediatric patients representing 1927.2 patient-years of therapy, no malignancies, including lymphoma or NMSC, have been reported.
5.4 New Onset or Worsening of Heart Failure
Two clinical trials evaluating the use of Enbrel in the treatment of heart failure were terminated early due to lack of efficacy. One of these studies suggested higher mortality in Enbrel-treated patients compared to placebo [see Adverse Reactions (6.2)]. There have been postmarketing reports of worsening of congestive heart failure (CHF), with and without identifiable precipitating factors, in patients taking Enbrel. There have also been rare (< 0.1%) reports of new onset CHF, including CHF in patients without known preexisting cardiovascular disease. Some of these patients have been under 50 years of age. Physicians should exercise caution when using Enbrel in patients who also have heart failure, and monitor patients carefully.
5.5 Hematologic Reactions
Rare (< 0.1%) reports of pancytopenia, including very rare (< 0.01%) reports of aplastic anemia, some with a fatal outcome, have been reported in patients treated with Enbrel. The causal relationship to Enbrel therapy remains unclear. Although no high-risk group has been identified, caution should be exercised in patients being treated with Enbrel who have a previous history of significant hematologic abnormalities. All patients should be advised to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever, bruising, bleeding, pallor) while on Enbrel. Discontinuation of Enbrel therapy should be considered in patients with confirmed significant hematologic abnormalities.
Two percent of patients treated concurrently with Enbrel and anakinra developed neutropenia (ANC < 1 × 109/L). While neutropenic, one patient developed cellulitis that resolved with antibiotic therapy.
5.6 Hepatitis B Reactivation
Reactivation of hepatitis B in patients who were previously infected with the hepatitis B virus (HBV) and had received concomitant TNF-blocking agents, including very rare cases (< 0.01%) with Enbrel, has been reported. In some instances, hepatitis B reactivation occurring in conjunction with TNF-blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to hepatitis B reactivation. Patients at risk for HBV infection should be evaluated for prior evidence of HBV infection before initiating TNF-blocker therapy. Prescribers should exercise caution in prescribing TNF-blockers in patients previously infected with HBV. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF-blocker therapy to prevent HBV reactivation. Patients previously infected with HBV and requiring treatment with Enbrel should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop HBV reactivation, consideration should be given to stopping Enbrel and initiating anti-viral therapy with appropriate supportive treatment. The safety of resuming Enbrel therapy after HBV reactivation is controlled is not known. Therefore, prescribers should weigh the risks and benefits when considering resumption of therapy in this situation.
5.7 Allergic Reactions
Allergic reactions associated with administration of Enbrel during clinical trials have been reported in < 2% of patients. If an anaphylactic reaction or other serious allergic reaction occurs, discontinue administration of Enbrel and initiate appropriate therapy immediately.
5.8 Immunizations
Avoid concurrent administration of live vaccines with Enbrel. It is recommended that patients, if possible, be brought up-to-date with all immunizations in agreement with current immunization guidelines prior to initiating Enbrel therapy [see Drug Interactions (7.1) and Use in Specific Populations (8.4)].
5.9 Autoimmunity
Treatment with Enbrel may result in the formation of autoantibodies [see Adverse Reactions (6.1)] and, rarely (< 0.1%), in the development of a lupus-like syndrome or autoimmune hepatitis [see Adverse Reactions (6.2)], which may resolve following withdrawal of Enbrel. If a patient develops symptoms and findings suggestive of a lupus-like syndrome or autoimmune hepatitis following treatment with Enbrel, discontinue treatment and evaluate the patient.
5.10 Immunosuppression
TNF mediates inflammation and modulates cellular immune responses. TNF-blocking agents, including Enbrel, affect host defenses against infections. The effect of TNF inhibition on the development and course of malignancies is not fully understood. In a study of 49 patients with RA treated with Enbrel, there was no evidence of depression of delayed-type hypersensitivity, depression of immunoglobulin levels, or change in enumeration of effector cell populations [see Warnings and Precautions (5.1, 5.3) and Adverse Reactions (6.1)].
5.11 Not Recommended for Use in Patients with Granulomatosis with Polyangiitis Receiving Immunosuppressants
The use of Enbrel in patients with granulomatosis with polyangiitis receiving immunosuppressive agents is not recommended. In a study of patients with granulomatosis with polyangiitis, the addition of Enbrel to standard therapy (including cyclophosphamide) was associated with a higher incidence of non-cutaneous solid malignancies and was not associated with improved clinical outcomes when compared with standard therapy alone [see Drug Interactions (7.3)].
5.12 Not Recommended for Use with Anakinra or Abatacept
Use of Enbrel with anakinra or abatacept is not recommended [see Drug Interactions (7.2)].
5.13 Increased Mortality in Patients with Moderate to Severe Alcoholic Hepatitis
In a study of 48 hospitalized patients treated with Enbrel or placebo for moderate to severe alcoholic hepatitis, the mortality rate in patients treated with Enbrel was similar to patients treated with placebo at 1 month but significantly higher after 6 months. Physicians should use caution when using Enbrel in patients with moderate to severe alcoholic hepatitis.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Serious Infections [see Warnings and Precautions (5.1)]
- Neurologic Reactions [see Warnings and Precautions (5.2)]
- Malignancies [see Warnings and Precautions (5.3)]
- Patients with Heart Failure [see Warnings and Precautions (5.4)]
- Hematologic Reactions [see Warnings and Precautions (5.5)]
- Hepatitis B Reactivation [see Warnings and Precautions (5.6)]
- Allergic Reactions [see Warnings and Precautions (5.7)]
- Autoimmunity [see Warnings and Precautions (5.9)]
- Immunosuppression [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Across clinical studies and postmarketing experience, the most serious adverse reactions with Enbrel were infections, neurologic events, CHF, and hematologic events [see Warnings and Precautions (5)]. The most common adverse reactions with Enbrel were infections and injection site reactions.
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not predict the rates observed in clinical practice.
Adverse Reactions in Adult Patients with Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, or Plaque Psoriasis
The data described below reflect exposure to Enbrel in 2219 adult patients with RA followed for up to 80 months, in 182 patients with PsA for up to 24 months, in 138 patients with AS for up to 6 months, and in 1204 adult patients with PsO for up to 18 months.
In controlled trials, the proportion of Enbrel-treated patients who discontinued treatment due to adverse events was approximately 4% in the indications studied.
Adverse Reactions in Pediatric Patients
In general, the adverse reactions in pediatric patients were similar in frequency and type as those seen in adult patients [see Warnings and Precautions (5), Use in Specific Populations (8.4), and Clinical Studies (14.2, 14.6)].
In a 48-week clinical study in 211 children aged 4 to 17 years with pediatric PsO, the adverse reactions reported were similar to those seen in previous studies in adults with PsO. Long-term safety profile for up to 264 additional weeks was assessed in an open-label extension study and no new safety signals were identified.
In open-label clinical studies of children with JIA, adverse reactions reported in those ages 2 to 4 years were similar to adverse reactions reported in older children.
Infections
Infections, including viral, bacterial, and fungal infections, have been observed in adult and pediatric patients. Infections have been noted in all body systems and have been reported in patients receiving Enbrel alone or in combination with other immunosuppressive agents.
In controlled portions of trials, the types and severity of infection were similar between Enbrel and the respective control group (placebo or MTX for RA and PsA patients) in RA, PsA, AS and PsO patients. Rates of infections in RA and adult PsO patients are provided in Table 3 and Table 4, respectively. Infections consisted primarily of upper respiratory tract infection, sinusitis and influenza.
In controlled portions of trials in RA, PsA, AS and PsO, the rates of serious infection were similar (0.8% in placebo, 3.6% in MTX, and 1.4% in Enbrel/Enbrel + MTX-treated groups). In clinical trials in rheumatologic indications, serious infections experienced by patients have included, but are not limited to, pneumonia, cellulitis, septic arthritis, bronchitis, gastroenteritis, pyelonephritis, sepsis, abscess and osteomyelitis. In clinical trials in adult PsO patients, serious infections experienced by patients have included, but are not limited to, pneumonia, cellulitis, gastroenteritis, abscess and osteomyelitis. The rate of serious infections was not increased in open-label extension trials and was similar to that observed in Enbrel- and placebo-treated patients from controlled trials.
In 66 global clinical trials of 17,505 patients (21,015 patient-years of therapy), tuberculosis was observed in approximately 0.02% of patients. In 17,696 patients (27,169 patient-years of therapy) from 38 clinical trials and 4 cohort studies in the U.S. and Canada, tuberculosis was observed in approximately 0.006% of patients. These studies include reports of pulmonary and extrapulmonary tuberculosis [see Warnings and Precautions (5.1)].
The types of infections reported in pediatric patients with PsO and JIA were generally mild and consistent with those commonly seen in the general pediatric population. Two JIA patients developed varicella infection and signs and symptoms of aseptic meningitis, which resolved without sequelae.
Injection Site Reactions
In placebo-controlled trials in rheumatologic indications, approximately 37% of patients treated with Enbrel developed injection site reactions. In controlled trials in patients with PsO, 15% of adult patients and 7% of pediatric patients treated with Enbrel developed injection site reactions during the first 3 months of treatment. All injection site reactions were described as mild to moderate (erythema, itching, pain, swelling, bleeding, bruising) and generally did not necessitate drug discontinuation. Injection site reactions generally occurred in the first month and subsequently decreased in frequency. The mean duration of injection site reactions was 3 to 5 days. Seven percent of patients experienced redness at a previous injection site when subsequent injections were given.
Other Adverse Reactions
Table 3 summarizes adverse reactions reported in adult RA patients. The types of adverse reactions seen in patients with PsA or AS were similar to the types of adverse reactions seen in patients with RA.
| Placebo-Controlled*
(Studies I, II, and a Phase 2 Study) | Active-Controlled†
(Study III) |
|||
|---|---|---|---|---|
| Placebo (N = 152) | Enbrel‡
(N = 349) | MTX (N = 217) | Enbrel‡
(N = 415) |
|
| Adverse Reaction | Percent of Patients | Percent of Patients | ||
|
||||
| Infection§ (total) | 39 | 50 | 86 | 81 |
| Upper Respiratory Infections¶ | 30 | 38 | 70 | 65 |
| Non-upper Respiratory Infections | 15 | 21 | 59 | 54 |
| Injection Site Reactions | 11 | 37 | 18 | 43 |
| Diarrhea | 9 | 8 | 16 | 16 |
| Rash | 2 | 3 | 19 | 13 |
| Pruritus | 1 | 2 | 5 | 5 |
| Pyrexia | - | 3 | 4 | 2 |
| Urticaria | 1 | - | 4 | 2 |
| Hypersensitivity | - | - | 1 | 1 |
In placebo-controlled adult PsO trials, the percentages of patients reporting adverse reactions in the 50 mg twice a week dose group were similar to those observed in the 25 mg twice a week dose group or placebo group.
Table 4 summarizes adverse reactions reported in adult PsO patients from Studies I and II.
| Placebo (N = 359) | Enbrel*
(N = 876) |
|
|---|---|---|
| Adverse Reaction | Percent of Patients | |
| Infection† (total) | 28 | 27 |
| Non-upper Respiratory Infections | 14 | 12 |
| Upper Respiratory Infections‡ | 17 | 17 |
| Injection Site Reactions | 6 | 15 |
| Diarrhea | 2 | 3 |
| Rash | 1 | 1 |
| Pruritus | 2 | 1 |
| Urticaria | - | 1 |
| Hypersensitivity | - | 1 |
| Pyrexia | 1 | - |
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to etanercept in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Immunogenicity
Patients with RA, PsA, AS or PsO were tested at multiple time points for antibodies to etanercept. Antibodies to the TNF receptor portion or other protein components of the Enbrel drug product were detected at least once in sera of approximately 6% of adult patients with RA, PsA, AS or PsO. These antibodies were all non-neutralizing. Results from JIA patients were similar to those seen in adult RA patients treated with Enbrel.
In adult PsO studies that evaluated the exposure of etanercept for up to 120 weeks, the percentage of patients testing positive at the assessed time points of 24, 48, 72 and 96 weeks ranged from 3.6%-8.7% and were all non-neutralizing. The percentage of patients testing positive increased with an increase in the duration of study; however, the clinical significance of this finding is unknown. No apparent correlation of antibody development to clinical response or adverse events was observed. The immunogenicity data of Enbrel beyond 120 weeks of exposure are unknown.
In pediatric PsO studies, approximately 10% of subjects developed antibodies to etanercept by Week 48 and approximately 16% of subjects developed antibodies to etanercept by Week 264. All of these antibodies were non-neutralizing. However, because of the limitations of the immunogenicity assays, the incidence of binding and neutralizing antibodies may not have been reliably determined.
The data reflect the percentage of patients whose test results were considered positive for antibodies to etanercept in an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay.
Autoantibodies
Patients with RA had serum samples tested for autoantibodies at multiple time points. In RA Studies I and II, the percentage of patients evaluated for antinuclear antibodies (ANA) who developed new positive ANA (titer ≥ 1:40) was higher in patients treated with Enbrel (11%) than in placebo-treated patients (5%). The percentage of patients who developed new positive anti-double-stranded DNA antibodies was also higher by radioimmunoassay (15% of patients treated with Enbrel compared to 4% of placebo-treated patients) and by Crithidia luciliae assay (3% of patients treated with Enbrel compared to none of placebo-treated patients). The proportion of patients treated with Enbrel who developed anticardiolipin antibodies was similarly increased compared to placebo-treated patients. In RA Study III, no pattern of increased autoantibody development was seen in Enbrel patients compared to MTX patients [see Warnings and Precautions (5.9)].
6.3 Postmarketing Experience
Adverse reactions have been reported during post approval use of Enbrel in adults and pediatric patients. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Enbrel exposure.
Adverse reactions are listed by body system below:
| Blood and lymphatic system disorders: | pancytopenia, anemia, leukopenia, neutropenia, thrombocytopenia, lymphadenopathy, aplastic anemia [see Warnings and Precautions (5.5)] |
| Cardiac disorders: | congestive heart failure [see Warnings and Precautions (5.4)] |
| Gastrointestinal disorders: | inflammatory bowel disease (IBD) |
| General disorders: | angioedema, chest pain |
| Hepatobiliary disorders: | autoimmune hepatitis, elevated transaminases, hepatitis B reactivation |
| Immune disorders: | macrophage activation syndrome, systemic vasculitis, sarcoidosis |
| Musculoskeletal and connective tissue disorders: | lupus-like syndrome |
| Neoplasms benign, malignant, and unspecified: | melanoma and non-melanoma skin cancers, Merkel cell carcinoma [see Warnings and Precautions (5.3)] |
| Nervous system disorders: | convulsions, multiple sclerosis, demyelination, optic neuritis, transverse myelitis, paresthesias, headache [see Warnings and Precautions (5.2)] |
| Ocular disorders: | uveitis, scleritis |
| Respiratory, thoracic and mediastinal disorders: | interstitial lung disease |
| Skin and subcutaneous tissue disorders: | cutaneous lupus erythematosus, cutaneous vasculitis (including leukocytoclastic vasculitis), erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, subcutaneous nodule, new or worsening psoriasis (all sub-types including pustular and palmoplantar) |
Opportunistic infections, including atypical mycobacterial infection, herpes zoster, aspergillosis and Pneumocystis jiroveci pneumonia, and protozoal infections have also been reported in postmarketing use.
Rare (< 0.1%) cases of IBD have been reported in JIA patients receiving Enbrel, which is not effective for the treatment of IBD.
7 DRUG INTERACTIONS
Specific drug interaction studies have not been conducted with Enbrel.
7.1 Vaccines
Most PsA patients receiving Enbrel were able to mount effective B-cell immune responses to pneumococcal polysaccharide vaccine, but titers in aggregate were moderately lower and fewer patients had 2-fold rises in titers compared to patients not receiving Enbrel. The clinical significance of this is unknown. Patients receiving Enbrel may receive concurrent vaccinations, except for live vaccines. No data are available on the secondary transmission of infection by live vaccines in patients receiving Enbrel.
Patients with a significant exposure to varicella virus should temporarily discontinue Enbrel therapy and be considered for prophylactic treatment with varicella zoster immune globulin [see Warnings and Precautions (5.8, 5.10)].
7.2 Immune-Modulating Biologic Products
In a study in which patients with active RA were treated for up to 24 weeks with concurrent Enbrel and anakinra therapy, a 7% rate of serious infections was observed, which was higher than that observed with Enbrel alone (0%) [see Warnings and Precautions (5.12)] and did not result in higher ACR response rates compared to Enbrel alone. The most common infections consisted of bacterial pneumonia (4 cases) and cellulitis (4 cases). One patient with pulmonary fibrosis and pneumonia died due to respiratory failure. Two percent of patients treated concurrently with Enbrel and anakinra developed neutropenia (ANC < 1 × 109/L).
In clinical studies, concurrent administration of abatacept and Enbrel resulted in increased incidences of serious adverse events, including infections, and did not demonstrate increased clinical benefit [see Warnings and Precautions (5.12)].
7.3 Cyclophosphamide
The use of Enbrel in patients receiving concurrent cyclophosphamide therapy is not recommended [see Warnings and Precautions (5.11)].
7.4 Sulfasalazine
Patients in a clinical study who were on established therapy with sulfasalazine, to which Enbrel was added, were noted to develop a mild decrease in mean neutrophil counts in comparison to groups treated with either Enbrel or sulfasalazine alone. The clinical significance of this observation is unknown.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available studies with use of etanercept during pregnancy do not reliably support an association between etanercept and major birth defects. Clinical data are available from the Organization of Teratology Information Specialists (OTIS) Enbrel Pregnancy Registry in women with rheumatic diseases or psoriasis and a Scandinavian study in pregnant women with chronic inflammatory disease. Both the OTIS Registry and the Scandinavian study showed the proportion of liveborn infants with major birth defects was higher for women exposed to etanercept compared to diseased etanercept unexposed women. However, the lack of pattern of major birth defects is reassuring and differences between exposure groups (e.g., disease severity) may have impacted the occurrence of birth defects (see Data). In animal reproduction studies with pregnant rats and rabbits, no fetal harm or malformations were observed with subcutaneous administration of etanercept during the period of organogenesis at doses that achieved systemic exposures 48 to 58 times the exposure in patients treated with 50 mg Enbrel once weekly (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the United States, about 2-4% of liveborn babies have a major birth defect and about 15-20% of pregnancies end in miscarriage, regardless of drug exposure.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
The risk of fetal/neonatal adverse reactions with in utero exposure to Enbrel is unknown. Risks and benefits should be considered prior to administering live or live-attenuated vaccines to infants exposed to Enbrel in utero [see Warnings and Precautions (5.8) and Drug Interactions (7.1)].
Data
Human Data
A prospective cohort pregnancy registry conducted by OTIS in the US and Canada between 2000 and 2012 compared the risk of major birth defects in liveborn infants of women with rheumatic diseases or psoriasis exposed to etanercept in the first trimester. The proportion of major birth defects among liveborn infants in the etanercept-exposed (N = 319) and diseased etanercept unexposed cohorts (N = 144) was 9.4% and 3.5%, respectively. The findings showed no statistically significant increased risk of minor birth defects and no pattern of major or minor birth defects.
A Scandinavian study compared the risk of major birth defects in liveborn infants of women with chronic inflammatory disease (CID) exposed to TNF-inhibitors during early pregnancy. Women were identified from the Danish (2004-2012) and Swedish (2006-2012) population-based health registers. The proportion of major birth defects among liveborn infants in the etanercept-exposed (N = 344) and CID etanercept unexposed cohorts (N = 21,549) was 7.0% and 4.7%, respectively.
Overall, while both the OTIS Registry and Scandinavian study show a higher proportion of major birth defects in etanercept-exposed patients compared to diseased etanercept unexposed patients, the lack of pattern of birth defects is reassuring and differences between exposure groups (e.g., disease severity) may have impacted the occurrence of birth defects.
Three case reports from the literature showed that cord blood levels of etanercept at delivery, in infants born to women administered etanercept during pregnancy, were between 3% and 32% of the maternal serum level.
Animal Data
In embryofetal development studies with etanercept administered during the period of organogenesis to pregnant rats from gestation day (GD) 6 through 20 or pregnant rabbits from GD 6 through 18, there was no evidence of fetal malformations or embryotoxicity in rats or rabbits at respective doses that achieved systemic exposures 48 to 58 times the exposure in patients treated with 50 mg Enbrel once weekly (on an AUC basis with maternal subcutaneous doses up to 30 mg/kg/day in rats and 40 mg/kg/day in rabbits). In a peri-and post-natal development study with pregnant rats that received etanercept during organogenesis and the later gestational period from GD 6 through 21, development of pups through post-natal day 4 was unaffected at doses that achieved exposures 48 times the exposure in patients treated with 50 mg Enbrel once weekly (on an AUC basis with maternal subcutaneous doses up to 30 mg/kg/day).
8.2 Lactation
Risk Summary
Limited data from published literature show that etanercept is present in low levels in human milk and minimally absorbed by a breastfed infant. No data are available on the effects of etanercept on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Enbrel and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.
8.4 Pediatric Use
Polyarticular Juvenile Idiopathic Arthritis
The safety and effectiveness of Enbrel have been established in pediatric patients 2 years of age and older with pJIA.
Enbrel has been studied in 69 children with moderately to severely active polyarticular JIA 2 to 17 years of age.
The safety and effectiveness of Enbrel in pediatric patients less than 2 years of age with pJIA have not been established.
Juvenile Psoriatic Arthritis
The safety and effectiveness of Enbrel have been established in pediatric patients 2 years to 17 years old with JPsA. Use of Enbrel in JPsA is supported by evidence from adequate and well controlled studies of Enbrel in adults with PsA; pharmacokinetic data from adult patients with PsA, RA, and PsO; and pharmacokinetic data from pediatric patients with active JIA and PsO. Safety of Enbrel in JPsA is supported by a clinical study in 69 pediatric patients with moderately to severely active JIA aged 2 to 17 years; a clinical study in 211 pediatric patients with moderate to severe PsO aged 4 to 17 years; and an open-label extension study in 182 pediatric patients with moderate to severe PsO aged 4 to 17 years.
The observed pre-dose (trough) concentrations are generally comparable between adults with RA and PsA and pediatric patients with active JIA, as well as adults with PsO and pediatric patients with PsO. The PK exposure is expected to be comparable between adults with PsA and pediatric patients with JPsA [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1, 14.2, 14.3, 14.5, 14.6)].
The safety and effectiveness in pediatric patients below the age of 2 years have not been established in JPsA.
Plaque Psoriasis
The safety and effectiveness of Enbrel for plaque psoriasis have been established in pediatric patients 4 years of age and older. Enbrel has been studied in 211 pediatric patients with moderate to severe PsO aged 4 to 17 years.
The safety and effectiveness of Enbrel in pediatric patients below the age of 4 years with PsO have not been established.
Malignancies in Pediatric Patients
Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy at ≤ 18 years of age), including Enbrel [see Warnings and Precautions (5.3)].
8.5 Geriatric Use
A total of 480 RA patients ages 65 years or older have been studied in clinical trials. In PsO randomized clinical trials, a total of 138 out of 1965 patients treated with Enbrel or placebo were age 65 or older. No overall differences in safety or effectiveness were observed between these patients and younger patients, but the number of geriatric PsO patients is too small to determine whether they respond differently from younger patients. Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly.
10 OVERDOSAGE
No dose-limiting toxicities have been observed during clinical trials of Enbrel. Single IV doses up to 60 mg/m2 (approximately twice the recommended dose) have been administered to healthy volunteers in an endotoxemia study without evidence of dose-limiting toxicities.
11 DESCRIPTION
Etanercept, a tumor necrosis factor (TNF) blocker, is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the human 75 kilodalton (p75) tumor necrosis factor receptor (TNFR) linked to the Fc portion of human IgG1. The Fc component of etanercept contains the CH2 domain, the CH3 domain and hinge region, but not the CH1 domain of IgG1. Etanercept is produced by recombinant DNA technology in a Chinese hamster ovary (CHO) mammalian cell expression system. It consists of 934 amino acids and has an apparent molecular weight of approximately 150 kilodaltons.
Enbrel (etanercept) Injection in the single-dose prefilled syringe, the single-dose prefilled SureClick autoinjector and the single-dose vial is clear and colorless, sterile, preservative-free solution, and is formulated at pH 6.3 ± 0.2.
Enbrel (etanercept) for Injection is supplied in a multiple-dose vial as a sterile, white, preservative-free, lyophilized powder. Reconstitution with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (containing 0.9% benzyl alcohol) yields a multiple-dose, clear, and colorless solution 1 mL containing 25 mg of Enbrel, with a pH of 7.4 ± 0.3.
Enbrel (etanercept) Injection in the Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector is clear and colorless, sterile, preservative-free solution, and is formulated at pH 6.3 ± 0.2.
| Presentation | Active Ingredient Content | Inactive Ingredients Content |
|---|---|---|
| Enbrel 50 mg prefilled syringe and SureClick autoinjector | 50 mg etanercept in 1 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
| Enbrel 25 mg prefilled syringe | 25 mg etanercept in 0.5 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
| Enbrel 25 mg single-dose vial | 25 mg etanercept in 0.5 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
| Enbrel 25 mg multiple-dose vial | After reconstitution, 25 mg etanercept in 1 mL | 40 mg mannitol 10 mg sucrose 1.2 mg tromethamine |
| Enbrel 50 mg Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector only | 50 mg etanercept in 1 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
TNF is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. It plays an important role in the inflammatory processes of RA, polyarticular JIA, PsA, and AS and the resulting joint pathology. In addition, TNF plays a role in the inflammatory process of PsO. Elevated levels of TNF are found in involved tissues and fluids of patients with RA, JIA, PsA, AS, and PsO.
Two distinct receptors for TNF (TNFRs), a 55 kilodalton protein (p55) and a 75 kilodalton protein (p75), exist naturally as monomeric molecules on cell surfaces and in soluble forms. Biological activity of TNF is dependent upon binding to either cell surface TNFR.
Etanercept is a dimeric soluble form of the p75 TNF receptor that can bind TNF molecules. Etanercept inhibits binding of TNF-α and TNF-β (lymphotoxin alpha [LT-α]) to cell surface TNFRs, rendering TNF biologically inactive. In in vitro studies, large complexes of etanercept with TNF-α were not detected and cells expressing transmembrane TNF (that binds Enbrel) are not lysed in the presence or absence of complement.
12.2 Pharmacodynamics
Etanercept can modulate biological responses that are induced or regulated by TNF, including expression of adhesion molecules responsible for leukocyte migration (e.g. E-selectin, and to a lesser extent, intercellular adhesion molecule-1 [ICAM-1]), serum levels of cytokines (e.g. IL-6), and serum levels of matrix metalloproteinase-3 (MMP-3 or stromelysin). Etanercept has been shown to affect several animal models of inflammation, including murine collagen-induced arthritis.
12.3 Pharmacokinetics
After administration of 25 mg of Enbrel by a single SC injection to 25 patients with RA, a mean ± standard deviation half-life of 102 ± 30 hours was observed with a clearance of 160 ± 80 mL/hr. A maximum serum concentration (Cmax) of 1.1 ± 0.6 mcg/mL and time to Cmax of 69 ± 34 hours was observed in these patients following a single 25 mg dose. After 6 months of twice weekly 25 mg doses in these same RA patients, the mean Cmax was 2.4 ± 1.0 mcg/mL (N = 23). Patients exhibited a 2- to 7-fold increase in peak serum concentrations and approximately 4-fold increase in AUC0-72 hr (range 1- to 17-fold) with repeated dosing. Serum concentrations in patients with RA have not been measured for periods of dosing that exceed 6 months.
In another study, serum concentration profiles at steady-state were comparable among patients with RA treated with 50 mg Enbrel once weekly and those treated with 25 mg Enbrel twice weekly. The mean (± standard deviation) Cmax, Cmin, and partial AUC were 2.4 ± 1.5 mcg/mL, 1.2 ± 0.7 mcg/mL, and 297 ± 166 mcg∙h/mL, respectively, for patients treated with 50 mg Enbrel once weekly (N = 21); and 2.6 ± 1.2 mcg/mL, 1.4 ± 0.7 mcg/mL, and 316 ± 135 mcg∙h/mL for patients treated with 25 mg Enbrel twice weekly (N = 16).
Patients with JIA (ages 4 to 17 years) were administered 0.4 mg/kg of Enbrel twice weekly (up to a maximum dose of 50 mg per week) for up to 18 weeks. The mean serum concentration after repeated SC dosing was 2.1 mcg/mL, with a range of 0.7 to 4.3 mcg/mL. Limited data suggest that the clearance of etanercept is reduced slightly in children ages 4 to 8 years. Population pharmacokinetic analyses predict that the pharmacokinetic differences between the regimens of 0.4 mg/kg twice weekly and 0.8 mg/kg once weekly in JIA patients are of the same magnitude as the differences observed between twice weekly and weekly regimens in adult RA patients.
The mean (± SD) serum steady-state trough concentrations for 50 mg QW dosing in adult PsA subjects were 2.1 ± 1.2 mcg/mL and 2.1 ± 1.4 mcg/mL at weeks 24 and 48, respectively.
The mean (± SD) serum steady-state trough concentrations for the 50 mg QW dosing in adult PsO subjects were 1.5 ± 0.7 mcg/mL. Pediatric PsO patients (age 4 to 17 years) were administered 0.8 mg/kg of Enbrel once weekly (up to a maximum dose of 50 mg per week) for up to 48 weeks. The mean (± SD) serum steady-state trough concentrations ranged from 1.6 ± 0.8 to 2.1 ± 1.3 mcg/mL at weeks 12, 24, and 48.
Overall, the observed etanercept concentrations in patients with JIA and pediatric PsO were within the range of those observed for adult RA, PsA and PsO after administration of Enbrel.
In clinical studies with Enbrel, pharmacokinetic parameters were not different between men and women and did not vary with age in adult patients. The pharmacokinetics of etanercept were unaltered by concomitant MTX in RA patients. No formal pharmacokinetic studies have been conducted to examine the effects of renal or hepatic impairment on etanercept disposition.
14 CLINICAL STUDIES
14.1 Adult Rheumatoid Arthritis
The safety and efficacy of Enbrel were assessed in four randomized, double-blind, controlled studies. The results of all four trials were expressed in percentage of patients with improvement in RA using ACR response criteria.
Study I evaluated 234 patients with active RA who were ≥ 18 years old, had failed therapy with at least one but no more than four disease-modifying antirheumatic drugs (DMARDs) (e.g. hydroxychloroquine, oral or injectable gold, MTX, azathioprine, D-penicillamine, sulfasalazine), and had ≥ 12 tender joints, ≥ 10 swollen joints, and either erythrocyte sedimentation rate (ESR) ≥ 28 mm/hr, C-reactive protein (CRP) > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg Enbrel or placebo were administered SC twice a week for 6 consecutive months.
Study II evaluated 89 patients and had similar inclusion criteria to Study I except that patients in Study II had additionally received MTX for at least 6 months with a stable dose (12.5 to 25 mg/week) for at least 4 weeks and they had at least 6 tender or painful joints. Patients in Study II received a dose of 25 mg Enbrel or placebo SC twice a week for 6 months in addition to their stable MTX dose.
Study III compared the efficacy of Enbrel to MTX in patients with active RA. This study evaluated 632 patients who were ≥ 18 years old with early (≤ 3 years disease duration) active RA, had never received treatment with MTX, and had ≥ 12 tender joints, ≥ 10 swollen joints, and either ESR ≥ 28 mm/hr, CRP > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg Enbrel were administered SC twice a week for 12 consecutive months. The study was unblinded after all patients had completed at least 12 months (and a median of 17.3 months) of therapy. The majority of patients remained in the study on the treatment to which they were randomized through 2 years, after which they entered an extension study and received open-label 25 mg Enbrel. MTX tablets (escalated from 7.5 mg/week to a maximum of 20 mg/week over the first 8 weeks of the trial) or placebo tablets were given once a week on the same day as the injection of placebo or Enbrel doses, respectively.
Study IV evaluated 682 adult patients with active RA of 6 months to 20 years duration (mean of 7 years) who had an inadequate response to at least one DMARD other than MTX. Forty-three percent of patients had previously received MTX for a mean of 2 years prior to the trial at a mean dose of 12.9 mg. Patients were excluded from this study if MTX had been discontinued for lack of efficacy or for safety considerations. The patient baseline characteristics were similar to those of patients in Study I. Patients were randomized to MTX alone (7.5 to 20 mg weekly, dose escalated as described for Study III; median dose 20 mg), Enbrel alone (25 mg twice weekly), or the combination of Enbrel and MTX initiated concurrently (at the same doses as above). The study evaluated ACR response, Sharp radiographic score, and safety.
Clinical Response
A higher percentage of patients treated with Enbrel and Enbrel in combination with MTX achieved ACR 20, ACR 50, and ACR 70 responses and Major Clinical Responses than in the comparison groups. The results of Studies I, II, and III are summarized in Table 6. The results of Study IV are summarized in Table 7.
| Placebo-Controlled | Active-Controlled | |||||
|---|---|---|---|---|---|---|
| Study I | Study II | Study III | ||||
| Placebo | Enbrel* | MTX/Placebo | MTX/Enbrel* | MTX | Enbrel* | |
| Response | N = 80 | N = 78 | N = 30 | N = 59 | N = 217 | N = 207 |
| ACR 20 | ||||||
| Month 3 | 23% | 62%† | 33% | 66%† | 56% | 62% |
| Month 6 | 11% | 59%† | 27% | 71%† | 58% | 65% |
| Month 12 | NA | NA | NA | NA | 65% | 72% |
| ACR 50 | ||||||
| Month 3 | 8% | 41%† | 0% | 42%† | 24% | 29% |
| Month 6 | 5% | 40%† | 3% | 39%† | 32% | 40% |
| Month 12 | NA | NA | NA | NA | 43% | 49% |
| ACR 70 | ||||||
| Month 3 | 4% | 15%† | 0% | 15%† | 7% | 13%‡ |
| Month 6 | 1% | 15%† | 0% | 15%† | 14% | 21%‡ |
| Month 12 | NA | NA | NA | NA | 22% | 25% |
| Endpoint | MTX (N = 228) | Enbrel (N = 223) | Enbrel/MTX (N = 231) |
|---|---|---|---|
|
|||
| ACR N*, † | |||
| Month 12 | 40% | 47% | 63%‡ |
| ACR 20 | |||
| Month 12 | 59% | 66% | 75%‡ |
| ACR 50 | |||
| Month 12 | 36% | 43% | 63%‡ |
| ACR 70 | |||
| Month 12 | 17% | 22% | 40%‡ |
| Major Clinical Response§ | 6% | 10% | 24%‡ |
The time course for ACR 20 response rates for patients receiving placebo or 25 mg Enbrel in Studies I and II is summarized in Figure 1. The time course of responses to Enbrel in Study III was similar.
|
|
Among patients receiving Enbrel, the clinical responses generally appeared within 1 to 2 weeks after initiation of therapy and nearly always occurred by 3 months. A dose response was seen in Studies I and III: 25 mg Enbrel was more effective than 10 mg (10 mg was not evaluated in Study II). Enbrel was significantly better than placebo in all components of the ACR criteria as well as other measures of RA disease activity not included in the ACR response criteria, such as morning stiffness.
In Study III, ACR response rates and improvement in all the individual ACR response criteria were maintained through 24 months of Enbrel therapy. Over the 2-year study, 23% of Enbrel patients achieved a major clinical response, defined as maintenance of an ACR 70 response over a 6-month period.
The results of the components of the ACR response criteria for Study I are shown in Table 8. Similar results were observed for Enbrel-treated patients in Studies II and III.
| Placebo N = 80 | Enbrel*
N = 78 |
|||
|---|---|---|---|---|
| Parameter (median) | Baseline | 3 Months | Baseline | 3 Months† |
|
||||
| Number of tender joints ‡ | 34.0 | 29.5 | 31.2 | 10.0§ |
| Number of swollen joints ¶ | 24.0 | 22.0 | 23.5 | 12.6§ |
| Physician global assessment # | 7.0 | 6.5 | 7.0 | 3.0§ |
| Patient global assessment # | 7.0 | 7.0 | 7.0 | 3.0§ |
| Pain # | 6.9 | 6.6 | 6.9 | 2.4§ |
| Disability index Þ | 1.7 | 1.8 | 1.6 | 1.0§ |
| ESR (mm/hr) | 31.0 | 32.0 | 28.0 | 15.5§ |
| CRP (mg/dL) | 2.8 | 3.9 | 3.5 | 0.9§ |
After discontinuation of Enbrel, symptoms of arthritis generally returned within a month. Reintroduction of treatment with Enbrel after discontinuations of up to 18 months resulted in the same magnitudes of response as in patients who received Enbrel without interruption of therapy, based on results of open-label studies.
Continued durable responses were seen for over 60 months in open-label extension treatment trials when patients received Enbrel without interruption. A substantial number of patients who initially received concomitant MTX or corticosteroids were able to reduce their doses or discontinue these concomitant therapies while maintaining their clinical responses.
Physical Function Response
In Studies I, II, and III, physical function and disability were assessed using the Health Assessment Questionnaire (HAQ). Additionally, in Study III, patients were administered the SF-36 Health Survey. In Studies I and II, patients treated with 25 mg Enbrel twice weekly showed greater improvement from baseline in the HAQ score beginning in month 1 through month 6 in comparison to placebo (p < 0.001) for the HAQ disability domain (where 0 = none and 3 = severe). In Study I, the mean improvement in the HAQ score from baseline to month 6 was 0.6 (from 1.6 to 1.0) for the 25 mg Enbrel group and 0 (from 1.7 to 1.7) for the placebo group. In Study II, the mean improvement from baseline to month 6 was 0.6 (from 1.5 to 0.9) for the Enbrel/MTX group and 0.2 (from 1.3 to 1.2) for the placebo/MTX group. In Study III, the mean improvement in the HAQ score from baseline to month 6 was 0.7 (from 1.5 to 0.7) for 25 mg Enbrel twice weekly. All subdomains of the HAQ in Studies I and III were improved in patients treated with Enbrel.
In Study III, patients treated with 25 mg Enbrel twice weekly showed greater improvement from baseline in SF-36 physical component summary score compared to Enbrel 10 mg twice weekly and no worsening in the SF-36 mental component summary score. In open-label Enbrel studies, improvements in physical function and disability measures have been maintained for up to 4 years.
In Study IV, median HAQ scores improved from baseline levels of 1.8, 1.8, and 1.8 to 1.1, 1.0, and 0.6 at 12 months in the MTX, Enbrel, and Enbrel/MTX combination treatment groups, respectively (combination versus both MTX and Enbrel, p < 0.01). Twenty-nine percent of patients in the MTX alone treatment group had an improvement of HAQ of at least 1 unit versus 40% and 51% in the Enbrel alone and the Enbrel/MTX combination treatment groups, respectively.
Radiographic Response
In Study III, structural joint damage was assessed radiographically and expressed as change in Total Sharp Score (TSS) and its components, the erosion score and Joint Space Narrowing (JSN) score. Radiographs of hands/wrists and forefeet were obtained at baseline, 6 months, 12 months, and 24 months and scored by readers who were unaware of treatment group. The results are shown in Table 9. A significant difference for change in erosion score was observed at 6 months and maintained at 12 months.
| MTX | 25 mg Enbrel | MTX/Enbrel (95% Confidence Interval*) | P Value | ||
|---|---|---|---|---|---|
|
|||||
| 12 Months | Total Sharp Score | 1.59 | 1.00 | 0.59 (-0.12, 1.30) | 0.1 |
| Erosion Score | 1.03 | 0.47 | 0.56 (0.11, 1.00) | 0.002 | |
| JSN Score | 0.56 | 0.52 | 0.04 (-0.39, 0.46) | 0.5 | |
| 6 Months | Total Sharp Score | 1.06 | 0.57 | 0.49 (0.06, 0.91) | 0.001 |
| Erosion Score | 0.68 | 0.30 | 0.38 (0.09, 0.66) | 0.001 | |
| JSN Score | 0.38 | 0.27 | 0.11 (-0.14, 0.35) | 0.6 | |
Patients continued on the therapy to which they were randomized for the second year of Study III. Seventy-two percent of patients had x-rays obtained at 24 months. Compared to the patients in the MTX group, greater inhibition of progression in TSS and erosion score was seen in the 25 mg Enbrel group, and, in addition, less progression was noted in the JSN score.
In the open-label extension of Study III, 48% of the original patients treated with 25 mg Enbrel have been evaluated radiographically at 5 years. Patients had continued inhibition of structural damage, as measured by the TSS, and 55% of them had no progression of structural damage. Patients originally treated with MTX had further reduction in radiographic progression once they began treatment with Enbrel.
In Study IV, less radiographic progression (TSS) was observed with Enbrel in combination with MTX compared with Enbrel alone or MTX alone at month 12 (Table 10). In the MTX treatment group, 55% of patients experienced no radiographic progression (TSS change ≤ 0.0) at 12 months compared to 63% and 76% in the Enbrel alone and the Enbrel/MTX combination treatment groups, respectively.
| MTX (N = 212)* | Enbrel (N = 212)* | Enbrel/MTX (N = 218)* |
|
|---|---|---|---|
| Total Sharp Score (TSS) | 2.80 (1.08, 4.51) | 0.52†
(-0.10, 1.15) | -0.54‡,§
(-1.00, -0.07) |
| Erosion Score (ES) | 1.68 (0.61, 2.74) | 0.21†
(-0.20, 0.61) | -0.30‡
(-0.65, 0.04) |
| Joint Space Narrowing (JSN) Score | 1.12 (0.34, 1.90) | 0.32 (0.00, 0.63) | -0.23‡,§
(-0.45, -0.02) |
Once Weekly Dosing
The safety and efficacy of 50 mg Enbrel (two 25 mg SC injections) administered once weekly were evaluated in a double-blind, placebo-controlled study of 420 patients with active RA. Fifty-three patients received placebo, 214 patients received 50 mg Enbrel once weekly, and 153 patients received 25 mg Enbrel twice weekly. The safety and efficacy profiles of the two Enbrel treatment groups were similar.
14.2 Polyarticular Juvenile Idiopathic Arthritis (JIA)
The safety and efficacy of Enbrel were assessed in a 2-part study in 69 children with polyarticular JIA who had a variety of JIA onset types. Patients ages 2 to 17 years with moderately to severely active polyarticular JIA refractory to or intolerant of MTX were enrolled; patients remained on a stable dose of a single nonsteroidal anti-inflammatory drug and/or prednisone (≤ 0.2 mg/kg/day or 10 mg maximum). In part 1, all patients received 0.4 mg/kg (maximum 25 mg per dose) Enbrel SC twice weekly. In part 2, patients with a clinical response at day 90 were randomized to remain on Enbrel or receive placebo for 4 months and assessed for disease flare. Responses were measured using the JIA Definition of Improvement (DOI), defined as ≥ 30% improvement in at least three of six and ≥ 30% worsening in no more than one of the six JIA core set criteria, including active joint count, limitation of motion, physician and patient/parent global assessments, functional assessment, and ESR. Disease flare was defined as a ≥ 30% worsening in three of the six JIA core set criteria and ≥ 30% improvement in not more than one of the six JIA core set criteria and a minimum of two active joints.
In part 1 of the study, 51 of 69 (74%) patients demonstrated a clinical response and entered part 2. In part 2, 6 of 25 (24%) patients remaining on Enbrel experienced a disease flare compared to 20 of 26 (77%) patients receiving placebo (p = 0.007). From the start of part 2, the median time to flare was ≥ 116 days for patients who received Enbrel and 28 days for patients who received placebo. Each component of the JIA core set criteria worsened in the arm that received placebo and remained stable or improved in the arm that continued on Enbrel. The data suggested the possibility of a higher flare rate among those patients with a higher baseline ESR. Of patients who demonstrated a clinical response at 90 days and entered part 2 of the study, some of the patients remaining on Enbrel continued to improve from month 3 through month 7, while those who received placebo did not improve.
The majority of JIA patients who developed a disease flare in part 2 and reintroduced Enbrel treatment up to 4 months after discontinuation re-responded to Enbrel therapy in open-label studies. Most of the responding patients who continued Enbrel therapy without interruption have maintained responses for up to 48 months.
Studies have not been done in patients with polyarticular JIA to assess the effects of continued Enbrel therapy in patients who do not respond within 3 months of initiating Enbrel therapy, or to assess the combination of Enbrel with MTX.
14.3 Psoriatic Arthritis
The safety and efficacy of Enbrel were assessed in a randomized, double-blind, placebo-controlled study in 205 patients with PsA. Patients were between 18 and 70 years of age and had active PsA (≥ 3 swollen joints and ≥ 3 tender joints) in one or more of the following forms: (1) distal interphalangeal (DIP) involvement (N = 104); (2) polyarticular arthritis (absence of rheumatoid nodules and presence of psoriasis; N = 173); (3) arthritis mutilans (N = 3); (4) asymmetric psoriatic arthritis (N = 81); or (5) ankylosing spondylitis-like (N = 7). Patients also had plaque psoriasis with a qualifying target lesion ≥ 2 cm in diameter. Patients on MTX therapy at enrollment (stable for ≥ 2 months) could continue at a stable dose of ≤ 25 mg/week MTX. Doses of 25 mg Enbrel or placebo were administered SC twice a week during the initial 6-month double-blind period of the study. Patients continued to receive blinded therapy in an up to 6-month maintenance period until all patients had completed the controlled period. Following this, patients received open-label 25 mg Enbrel twice a week in a 12-month extension period.
Compared to placebo, treatment with Enbrel resulted in significant improvements in measures of disease activity (Table 11).
| Placebo N = 104 | Enbrel*
N = 101 |
|||
|---|---|---|---|---|
| Parameter (median) | Baseline | 6 Months | Baseline | 6 Months |
|
||||
| Number of tender joints† | 17.0 | 13.0 | 18.0 | 5.0 |
| Number of swollen joints‡ | 12.5 | 9.5 | 13.0 | 5.0 |
| Physician global assessment§ | 3.0 | 3.0 | 3.0 | 1.0 |
| Patient global assessment§ | 3.0 | 3.0 | 3.0 | 1.0 |
| Morning stiffness (minutes) | 60 | 60 | 60 | 15 |
| Pain§ | 3.0 | 3.0 | 3.0 | 1.0 |
| Disability index¶ | 1.0 | 0.9 | 1.1 | 0.3 |
| CRP (mg/dL)# | 1.1 | 1.1 | 1.6 | 0.2 |
Among patients with PsA who received Enbrel, the clinical responses were apparent at the time of the first visit (4 weeks) and were maintained through 6 months of therapy. Responses were similar in patients who were or were not receiving concomitant MTX therapy at baseline. At 6 months, the ACR 20/50/70 responses were achieved by 50%, 37%, and 9%, respectively, of patients receiving Enbrel, compared to 13%, 4%, and 1%, respectively, of patients receiving placebo. Similar responses were seen in patients with each of the subtypes of PsA, although few patients were enrolled with the arthritis mutilans and ankylosing spondylitis-like subtypes. The results of this study were similar to those seen in an earlier single-center, randomized, placebo-controlled study of 60 patients with PsA.
The skin lesions of psoriasis were also improved with Enbrel, relative to placebo, as measured by percentages of patients achieving improvements in the Psoriasis Area and Severity Index (PASI). Responses increased over time, and at 6 months, the proportions of patients achieving a 50% or 75% improvement in the PASI were 47% and 23%, respectively, in the Enbrel group (N = 66), compared to 18% and 3%, respectively, in the placebo group (N = 62). Responses were similar in patients who were or were not receiving concomitant MTX therapy at baseline.
Radiographic Response
Radiographic changes were also assessed in the PsA study. Radiographs of hands and wrists were obtained at baseline and months 6, 12, and 24. A modified Total Sharp Score (TSS), which included distal interphalangeal joints (i.e., not identical to the modified TSS used for RA) was used by readers blinded to treatment group to assess the radiographs. Some radiographic features specific to PsA (e.g. pencil-and-cup deformity, joint space widening, gross osteolysis, and ankylosis) were included in the scoring system, but others (e.g. phalangeal tuft resorption, juxta-articular and shaft periostitis) were not.
Most patients showed little or no change in the modified TSS during this 24-month study (median change of 0 in both patients who initially received Enbrel or placebo). More placebo-treated patients experienced larger magnitudes of radiographic worsening (increased TSS) compared to Enbrel treatment during the controlled period of the study. At 12 months, in an exploratory analysis, 12% (12 of 104) of placebo patients compared to none of the 101 Enbrel-treated patients had increases of 3 points or more in TSS. Inhibition of radiographic progression was maintained in patients who continued on Enbrel during the second year. Of the patients with 1-year and 2-year x-rays, 3% (2 of 71) had increases of 3 points or more in TSS at 1 and 2 years.
Physical Function Response
In the PsA study, physical function and disability were assessed using the HAQ Disability Index (HAQ-DI) and the SF-36 Health Survey. Patients treated with 25 mg Enbrel twice weekly showed greater improvement from baseline in the HAQ-DI score (mean decreases of 54% at both months 3 and 6) in comparison to placebo (mean decreases of 6% at both months 3 and 6) (p < 0.001). At months 3 and 6, patients treated with Enbrel showed greater improvement from baseline in the SF-36 physical component summary score compared to patients treated with placebo, and no worsening in the SF-36 mental component summary score. Improvements in physical function and disability measures were maintained for up to 2 years through the open-label portion of the study.
14.4 Ankylosing Spondylitis
The safety and efficacy of Enbrel were assessed in a randomized, double-blind, placebo-controlled study in 277 patients with active AS. Patients were between 18 and 70 years of age and had AS as defined by the modified New York Criteria for Ankylosing Spondylitis. Patients were to have evidence of active disease based on values of ≥ 30 on a 0-100 unit Visual Analog Scale (VAS) for the average of morning stiffness duration and intensity, and two of the following three other parameters: a) patient global assessment, b) average of nocturnal and total back pain, and c) the average score on the Bath Ankylosing Spondylitis Functional Index (BASFI). Patients with complete ankylosis of the spine were excluded from study participation. Patients taking hydroxychloroquine, sulfasalazine, methotrexate, or prednisone (≤ 10 mg/day) could continue these drugs at stable doses for the duration of the study. Doses of 25 mg Enbrel or placebo were administered SC twice a week for 6 months.
The primary measure of efficacy was a 20% improvement in the Assessment in Ankylosing Spondylitis (ASAS) response criteria. Compared to placebo, treatment with Enbrel resulted in improvements in the ASAS and other measures of disease activity (Figure 2 and Table 12).
| Figure 2. ASAS 20 Responses in Ankylosing Spondylitis |
|---|
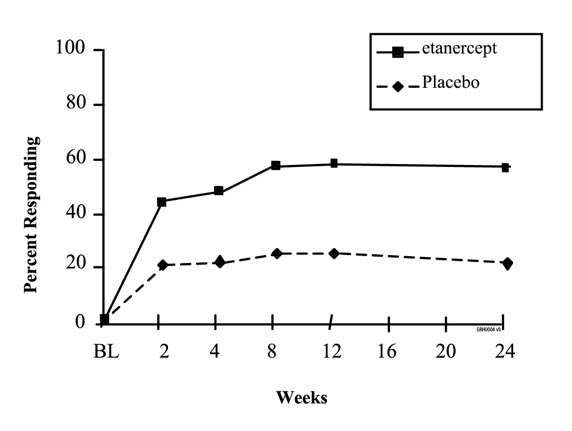 |
At 12 weeks, the ASAS 20/50/70 responses were achieved by 60%, 45%, and 29%, respectively, of patients receiving Enbrel, compared to 27%, 13%, and 7%, respectively, of patients receiving placebo (p ≤ 0.0001, Enbrel versus placebo). Similar responses were seen at Week 24. Responses were similar between those patients receiving concomitant therapies at baseline and those who were not. The results of this study were similar to those seen in a single-center, randomized, placebo-controlled study of 40 patients and a multicenter, randomized, placebo-controlled study of 84 patients with AS.
| Placebo N = 139 | Enbrel*
N = 138 |
|||
|---|---|---|---|---|
| Median values at time points | Baseline | 6 Months | Baseline | 6 Months |
|
||||
| ASAS response criteria | ||||
| Patient global assessment † | 63 | 56 | 63 | 36 |
| Back pain ‡ | 62 | 56 | 60 | 34 |
| BASFI § | 56 | 55 | 52 | 36 |
| Inflammation ¶ | 64 | 57 | 61 | 33 |
| Acute phase reactants | ||||
| CRP (mg/dL) # | 2.0 | 1.9 | 1.9 | 0.6 |
| Spinal mobility (cm): | ||||
| Modified Schober's test | 3.0 | 2.9 | 3.1 | 3.3 |
| Chest expansion | 3.2 | 3.0 | 3.3 | 3.9 |
| Occiput-to-wall measurement | 5.3 | 6.0 | 5.6 | 4.5 |
14.5 Adult Plaque Psoriasis
The safety and efficacy of Enbrel were assessed in two randomized, double-blind, placebo-controlled studies in adults with chronic stable PsO involving ≥ 10% of the body surface area, a minimum Psoriasis Area and Severity Index (PASI) score of 10 and who had received or were candidates for systemic antipsoriatic therapy or phototherapy. Patients with guttate, erythrodermic, or pustular psoriasis and patients with severe infections within 4 weeks of screening were excluded from study. No concomitant major antipsoriatic therapies were allowed during the study.
Study I evaluated 672 subjects who received placebo or Enbrel SC at doses of 25 mg once a week, 25 mg twice a week, or 50 mg twice a week for 3 months. After 3 months, subjects continued on blinded treatments for an additional 3 months during which time subjects originally randomized to placebo began treatment with blinded Enbrel at 25 mg twice weekly (designated as placebo/Enbrel in Table 13); subjects originally randomized to Enbrel continued on the originally randomized dose (designated as Enbrel/Enbrel groups in Table 13).
Study II evaluated 611 subjects who received placebo or Enbrel SC at doses of 25 mg or 50 mg twice a week for 3 months. After 3 months of randomized, blinded treatment, subjects in all three arms began receiving open-label Enbrel at 25 mg twice weekly for 9 additional months.
Response to treatment in both studies was assessed after 3 months of therapy and was defined as the proportion of subjects who achieved a reduction in PASI score of at least 75% from baseline. The PASI is a composite score that takes into consideration both the fraction of body surface area affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema and scaling).
Other evaluated outcomes included the proportion of subjects who achieved a score of "clear" or "minimal" by the Static Physician Global Assessment (sPGA) and the proportion of subjects with a reduction of PASI of at least 50% from baseline. The sPGA is a 6-category scale ranging from "5 = severe" to "0 = none" indicating the physician's overall assessment of the PsO severity focusing on induration, erythema and scaling. Treatment success of "clear" or "minimal" consisted of none or minimal elevation in plaque, up to faint red coloration in erythema and none or minimal fine scale over < 5% of the plaque.
Subjects in all treatment groups and in both studies had a median baseline PASI score ranging from 15 to 17, and the percentage of subjects with baseline sPGA classifications ranged from 54% to 66% for moderate, 17% to 26% for marked and 1% to 5% for severe. Across all treatment groups, the percentage of subjects who previously received systemic therapy for PsO ranged from 61% to 65% in Study I and 71% to 75% in Study II, and those who previously received phototherapy ranged from 44% to 50% in Study I and 72% to 73% in Study II.
More subjects randomized to Enbrel than placebo achieved at least a 75% reduction from baseline PASI score (PASI 75) with a dose response relationship across doses of 25 mg once a week, 25 mg twice a week and 50 mg twice a week (Tables 13 and 14). The individual components of the PASI (induration, erythema and scaling) contributed comparably to the overall treatment-associated improvement in PASI.
| Placebo/Enbrel | Enbrel/Enbrel | |||
|---|---|---|---|---|
| 25 mg BIW | 25 mg QW | 25 mg BIW | 50 mg BIW | |
| (N = 168) | (N = 169) | (N = 167) | (N = 168) | |
| 3 Months | ||||
| PASI 75 n (%) | 6 (4%) | 23 (14%)* | 53 (32%)† | 79 (47%)† |
| Difference (95% CI) | 10% (4, 16) | 28% (21, 36) | 43% (35, 52) | |
| sPGA, "clear" or "minimal" n (%) | 8 (5%) | 36 (21%)† | 53 (32%)† | 79 (47%)† |
| Difference (95% CI) | 17% (10, 24) | 27% (19, 35) | 42% (34, 50) | |
| PASI 50 n (%) | 24 (14%) | 62 (37%)† | 90 (54%)† | 119 (71%)† |
| Difference (95% CI) | 22% (13, 31) | 40% (30, 49) | 57% (48, 65) | |
| 6 Months | ||||
| PASI 75 n (%) | 55 (33%) | 36 (21%) | 68 (41%) | 90 (54%) |
| Placebo | Enbrel | ||
|---|---|---|---|
| 25 mg BIW | 50 mg BIW | ||
| (N = 204) | (N = 204) | (N = 203) | |
|
|||
| PASI 75 n (%) | 6 (3%) | 66 (32%)* | 94 (46%)* |
| Difference (95% CI) | 29% (23, 36) | 43% (36, 51) | |
| sPGA, "clear" or "minimal" n (%) | 7 (3%) | 75 (37%)* | 109 (54%)* |
| Difference (95% CI) | 34% (26, 41) | 50% (43, 58) | |
| PASI 50 n (%) | 18 (9%) | 124 (61%)* | 147 (72%)* |
| Difference (95% CI) | 52% (44, 60) | 64% (56, 71) | |
Among PASI 75 achievers in both studies, the median time to PASI 50 and PASI 75 was approximately 1 month and approximately 2 months, respectively, after the start of therapy with either 25 or 50 mg twice a week.
In Study I, subjects who achieved PASI 75 at month 6 were entered into a study drug withdrawal and retreatment period. Following withdrawal of study drug, these subjects had a median duration of PASI 75 of between 1 and 2 months.
In Study I, among subjects who were PASI 75 responders at 3 months, retreatment with their original blinded Enbrel dose after discontinuation of up to 5 months resulted in a similar proportion of responders as in the initial double-blind portion of the study.
In Study II, most subjects initially randomized to 50 mg twice a week continued in the study after month 3 and had their Enbrel dose decreased to 25 mg twice a week. Of the 91 subjects who were PASI 75 responders at month 3, 70 (77%) maintained their PASI 75 response at month 6.
14.6 Pediatric Plaque Psoriasis
A 48-week, randomized, double-blind, placebo-controlled study enrolled 211 pediatric subjects 4 to 17 years of age, with moderate to severe plaque psoriasis (PsO) (as defined by a sPGA score ≥ 3 [moderate, marked, or severe], involving ≥ 10% of the body surface area, and a PASI score ≥ 12) who were candidates for phototherapy or systemic therapy, or were inadequately controlled on topical therapy. Subjects in all treatment groups had a median baseline PASI score of 16.4, and the percentage of subjects with baseline sPGA classifications was 65% for moderate, 31% for marked, and 3% for severe. Across all treatment groups, the percentage of subjects who previously received systemic or phototherapy for PsO was 57%.
Subjects received Enbrel 0.8 mg/kg (up to a maximum of 50 mg per dose) or placebo once weekly for the first 12 weeks. After 12 weeks, subjects entered a 24-week open-label treatment period, in which all subjects received Enbrel at the same dose. This was followed by a 12-week withdrawal-retreatment period.
Response to treatment was assessed after 12 weeks of therapy and was defined as the proportion of subjects who achieved a reduction in PASI score of at least 75% from baseline. The PASI is a composite score that takes into consideration both the fraction of body surface area affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema and scaling).
Other evaluated outcomes included the proportion of subjects who achieved a score of "clear" or "almost clear" by the sPGA and the proportion of subjects with a reduction in PASI score of at least 90% from baseline. The sPGA is a 6-category scale ranging from "5 = severe" to "0 = none" indicating the physician's overall assessment of the PsO severity focusing on induration, erythema and scaling. Treatment success of "clear" or "almost clear" consisted of none or minimal elevation in plaque, up to faint red coloration in erythema and none or minimal fine scale over < 5% of the plaque.
Efficacy results are summarized in Table 15.
| Placebo (N = 105) | Enbrel 0.8 mg/kg Once Weekly (N = 106) |
|
|---|---|---|
| PASI 75, n (%) | 12 (11%) | 60 (57%) |
| PASI 90, n (%) | 7 (7%) | 29 (27%) |
| sPGA "clear" or "almost clear" n (%) | 14 (13%) | 55 (52%) |
Maintenance of Response
To evaluate maintenance of response, subjects who achieved PASI 75 response at Week 36 were re-randomized to either Enbrel or placebo during a 12-week randomized withdrawal period. The maintenance of PASI 75 response was evaluated at Week 48. The proportion of subjects who maintained PASI 75 response at Week 48 was higher for subjects treated with Enbrel (65%) compared to those treated with placebo (49%).
15 REFERENCES
- National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 13 Registries, 1992-2002.
- Bröms G, Granath F, Ekbom A, et al. Low Risk of Birth Defects for Infants Whose Mothers Are Treated With Anti-Tumor Necrosis Factor Agents During Pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.e5
16 HOW SUPPLIED/STORAGE AND HANDLING
Enbrel (etanercept) injection is supplied as a clear and colorless sterile, preservative-free solution for subcutaneous administration in single-dose prefilled syringes, an Enbrel single-dose prefilled SureClick autoinjector with a 27-gauge, ½-inch needle, or a single-dose vial. The prefilled syringe and SureClick autoinjector are not made with natural rubber latex.
Each Enbrel® Mini single-dose prefilled cartridge for use with the AutoTouch® reusable autoinjector contains 1.0 mL of 50 mg/mL of etanercept. The AutoTouch reusable autoinjector and Enbrel Mini single-dose prefilled cartridge are not made with natural rubber latex.
The AutoTouch reusable autoinjector contains no drug and must use an Enbrel Mini single-dose prefilled cartridge. In addition, the AutoTouch Connect® reusable autoinjector would allow for data connectivity via Bluetooth wireless technology.
| 50 mg/mL single-dose prefilled syringe | Carton of 4 | NDC 58406-435-04 NDC 58406-021-04 |
| 50 mg/mL single-dose prefilled SureClick autoinjector | Carton of 4 | NDC 58406-445-04 NDC 58406-032-04 |
| 25 mg/0.5 mL single-dose prefilled syringe | Carton of 4 | NDC 58406-455-04 NDC 58406-010-04 |
| 50 mg/mL Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector only | Cartridges: Carton of 4 | NDC 58406-456-04 NDC 58406-044-04 |
| AutoTouch Reusable Autoinjector: Carton of 1 | NDC 58406-470-01 | |
| AutoTouch Connect Reusable Autoinjector: Carton of 1 | NDC 58406-480-01 | |
| 25 mg/0.5 mL single-dose vial | Carton of 4 | NDC 58406-055-04 |
Enbrel should be refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light or physical damage. Do not store Enbrel in extreme heat or cold. DO NOT SHAKE. DO NOT FREEZE.
For convenience, storage of individual single-dose prefilled syringes, SureClick autoinjectors, single-dose vials, or Enbrel Mini cartridges at room temperature at 68°F to 77°F (20°C to 25°C) for a maximum single period of 30 days is permissible, with protection from light and sources of heat. Once a single-dose prefilled syringe, SureClick autoinjector, single-dose vial, or Enbrel Mini cartridge has been stored at room temperature, it should not be placed back into the refrigerator. If not used within 30 days at room temperature, the single-dose prefilled syringe, SureClick autoinjector, single-dose vial, or Enbrel Mini cartridge should be discarded.
Do not use Enbrel beyond the expiration date stamped on the carton or barrel/cartridge label. Keep out of the reach of children.
The AutoTouch reusable autoinjector should be stored at room temperature. Do not refrigerate the AutoTouch reusable autoinjector.
Enbrel Lyophilized Powder (Used for Weight-based Dosing)
Enbrel (etanercept) for Injection is supplied as lyophilized powder for reconstitution in a multiple-dose vial. Each vial is supplied in a carton containing four dose trays. Each dose tray contains one 25 mg vial of etanercept lyophilized powder, one diluent syringe (1 mL Sterile Bacteriostatic Water for Injection, USP, containing 0.9% benzyl alcohol), one 27-gauge ½-inch needle, one vial adapter, and one plunger. Each carton contains four "Mixing Date:" stickers.
| 25 mg multiple-dose vial | Carton of 4 | NDC 58406-425-34 |
Enbrel should be refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light or physical damage. Do not store Enbrel in extreme heat or cold. DO NOT SHAKE. DO NOT FREEZE.
For convenience, storage of an individual dose tray containing Enbrel multiple-dose vial and diluent syringe at room temperature at 68°F to 77°F (20°C to 25°C) for a maximum single period of 14 days is permissible, with protection from light, sources of heat, and humidity. Once the dose tray has been stored at room temperature, it should not be placed back into the refrigerator. If not used within 14 days at room temperature, the dose tray should be discarded. Once a vial has been reconstituted, the solution must be used immediately or may be refrigerated for up to 14 days.
Do not use Enbrel beyond the expiration date stamped on the dose tray. Keep out of the reach of children.
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use) before the patient starts using Enbrel, and each time the prescription is renewed, as there may be new information they need to know.
Patients or their caregivers should be provided the Enbrel "Medication Guide" and provided an opportunity to read it and ask questions prior to initiation of therapy. The healthcare provider should ask the patient questions to determine any risk factors for treatment. Patients developing signs and symptoms of infection should seek medical evaluation immediately.
Patient Counseling
Patients should be advised of the potential benefits and risks of Enbrel. Physicians should instruct their patients to read the Medication Guide before starting Enbrel therapy and to reread each time the prescription is renewed.
Infections
Inform patients that Enbrel may lower the ability of their immune system to fight infections. Advise patients of the importance of contacting their doctor if they develop any symptoms of infection, tuberculosis or reactivation of hepatitis B virus infections.
Other Medical Conditions
Advise patients to report any signs of new or worsening medical conditions, such as central nervous system demyelinating disorders, heart failure or autoimmune disorders, such as lupus-like syndrome or autoimmune hepatitis. Counsel about the risk of lymphoma and other malignancies while receiving Enbrel. Advise patients to report any symptoms suggestive of a pancytopenia, such as bruising, bleeding, persistent fever or pallor.
Administration of Enbrel
If a patient or caregiver is to administer Enbrel, the patient or caregiver should be instructed in injection techniques and how to measure and administer the correct dose [see "Instructions for Use"]. For weight-based dosing, instruct caregivers and patients on the proper techniques for preparing, storing, measuring, and administering Enbrel solution in a single-dose vial or reconstituted lyophilized powder in a multiple-dose vial.
The first injection should be performed under the supervision of a qualified healthcare professional. The patient's or caregiver's ability to inject subcutaneously should be assessed. Patients and caregivers should be instructed in the technique, as well as proper syringe and needle disposal, and be cautioned against reuse of needles and syringes.
When using the SureClick autoinjector to administer Enbrel, the patient or caregiver should be informed that the window turns yellow when the injection is complete. After removing the autoinjector, if the window has not turned yellow, or if it looks like the medicine is still injecting, this means the patient has not received a full dose. The patient or caregiver should be advised to call their healthcare provider immediately.
When using the AutoTouch reusable autoinjector to administer Enbrel, the patient or caregiver should be informed that the status button turns green upon contact with the skin, flashes green after starting the injection, and turns off at completion of the injection. After removing the AutoTouch reusable autoinjector from the skin, if the status button has turned red, the patient or caregiver should be advised to call 1-888-4Enbrel (1-888-436-2735) immediately. If it looks like the medicine is still injecting or there is still fluid in Enbrel Mini, this means the patient has not received a full dose. The patient or caregiver should be advised to call their healthcare provider immediately.
A puncture-resistant container for disposal of needles, syringes, SureClick autoinjectors, single-dose vials, and Enbrel Mini cartridges should be used. If the product is intended for multiple use, additional syringes, needles and alcohol swabs will be required.
Patients can be advised to call 1-888-4ENBREL (1-888-436-2735) or visit www.enbrel.com for more information about Enbrel.
AMGEN®
Enbrel® (etanercept)
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
U.S. License Number 1132
Patent: http://pat.amgen.com/enbrel/
© 1998-2023 Immunex Corporation. All rights reserved.
1XXXXXX – v70
 This printed material is recyclable.
This printed material is recyclable.
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 10/2023 | ||
| Medication Guide | |||
| Enbrel® (en-brel) (etanercept) injection, for subcutaneous use | Enbrel® (en-brel) (etanercept) for injection, for subcutaneous use |
||
| Read the Medication Guide that comes with Enbrel before you start using it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment. It is important to remain under your healthcare provider's care while using Enbrel. Enbrel is a prescription medicine called a Tumor Necrosis Factor (TNF) blocker that affects your immune system. |
|||
|
What is the most important information I should know about Enbrel?
2. Risk of cancer
Before starting Enbrel, be sure to talk to your healthcare provider: Enbrel may not be right for you. Before starting Enbrel, tell your healthcare provider about all of your medical conditions, including:Infections. Tell your healthcare provider if you:
Other important medical information you should tell your healthcare provider before starting Enbrel, includes if you:
|
|||
| What is Enbrel?
Enbrel is a prescription medicine called a Tumor Necrosis Factor (TNF) blocker. Enbrel is used to treat:
Enbrel can help reduce joint damage and the signs and symptoms of the above-mentioned diseases. People with these diseases have too much of a protein called tumor necrosis factor (TNF), which is made by your immune system. Enbrel can reduce the effect of TNF in the body and block the damage that too much TNF can cause, but it can also lower the ability of your immune system to fight infections. See "What is the most important information I should know about Enbrel?" and "What are the possible side effects of Enbrel?" |
|||
| Who should not use Enbrel? Do not use Enbrel if you:
|
|||
How should I use Enbrel?
|
|||
|
What are the possible side effects of Enbrel?
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
How should I store Enbrel?
|
|||
| General information about the safe and effective use of Enbrel.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Enbrel for a condition for which it was not prescribed. Do not give Enbrel to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about Enbrel. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Enbrel that was written for health professionals. |
|||
| What are the ingredients in Enbrel? Single-dose Prefilled Syringe, Single-dose Prefilled SureClick Autoinjector, Single-dose Vial and Enbrel Mini single-dose cartridge: Active Ingredient: etanercept Inactive Ingredients: L-arginine hydrochloride, sodium chloride, and sucrose Multiple-dose Vial: Active Ingredient: etanercept Inactive Ingredients: mannitol, sucrose, tromethamine AMGEN® Manufactured by: Immunex Corporation, Thousand Oaks, CA 91320-1799, U.S. License Number 1132 Immunex Corporation. All rights reserved. 1XXXXXX – v22 For more information, call 1 888 4ENBREL (1 888 436 2735) or www.enbrel.com. |
|||
|
|
|||
Instructions for Use
Enbrel® (en-brel)
(etanercept)
injection, for subcutaneous use
Single-Dose Prefilled SureClick® Autoinjector
50 mg/mL
This Instructions for Use contains information on how to inject ENBREL.
| Guide to parts |
| Before use | After use |
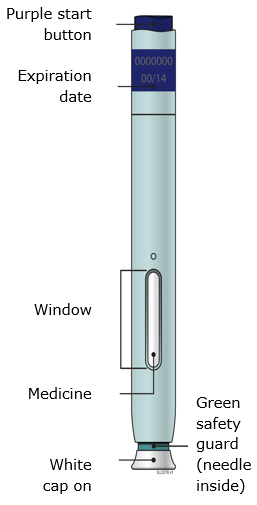 | 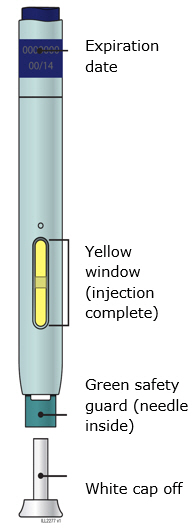 |
| Important: Needle is inside the green safety guard. | |
| Important |
Important Information You Need to Know Before Injecting ENBREL:
|
| Step 1: Preparing to inject ENBREL |
1A Remove the autoinjector from the carton.
|
|
| 30 minutes |
|
|
1B Inspect the Enbrel SureClick autoinjector.
|
|
| 1C Gather all materials needed for your injection.
Wash your hands thoroughly with soap and water. On a clean, well-lit work surface, place the:
|
|
1D Prepare and clean your injection site.
|
|
| Step 2: Get ready |
2A Pull the white cap straight off only when you are ready to inject.
|
|
|
|
|
|
|
| 2B Create a firm surface at the selected injection site (thigh, stomach, or outer areas of the upper arm), by using either the Stretch method or the Pinch method. Stretch method:  Stretch the skin firmly by moving your thumb and fingers in opposite directions, creating an area about 2 inches wide.  Pinch method:  Pinch the skin firmly between your thumb and fingers, creating an area about 2 inches wide. |
|
| Important: Keep skin stretched or pinched while injecting. |
| Step 3: Inject |
| 3A Keep holding the stretched or pinched skin. With the white cap off, put the green safety guard on your skin at 90 degrees. The needle is inside the green safety guard. Do not touch the purple start button yet. |
|
|
3B Firmly push down the autoinjector onto the skin until it stops moving. Important: You must push all the way down but do not touch the purple start button until you are ready to inject. |
3C When you are ready to inject, press the purple start button. You will hear a click.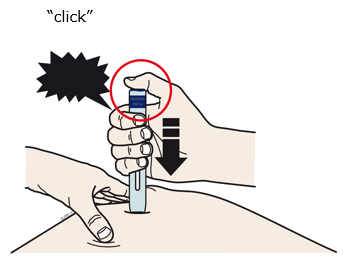 |
||||
| 3D Keep pushing the autoinjector down on your skin. Then lift your thumb while still holding the autoinjector on your skin. Your injection could take about 15 seconds to complete. | ||||
|
| 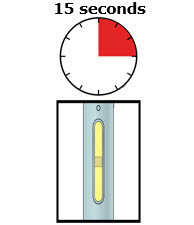 Window turns from clear to yellow when the injection is done. You may hear a second click. Window turns from clear to yellow when the injection is done. You may hear a second click. | |||
|
| NOTE: After you remove the autoinjector from your skin, the needle will be automatically covered. | |||
| Important: When you remove the autoinjector, if the window has not turned yellow, or if it looks like the medicine is still injecting, this means you have not received a full dose. Call your healthcare provider immediately. |
| Step 4: Finish |
| 4A Throw away the used autoinjector and white needle cap. | |
| 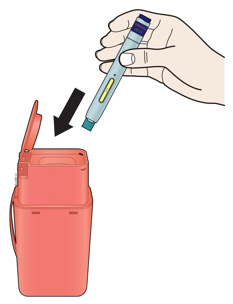 |
| When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
|
|
| Important: Always keep the sharps disposal container out of the reach of children. | |
4B Check the injection site.
|
|
| Commonly Asked Questions | |
|---|---|
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. | |
| What will happen if I press the purple start button before I am ready to do the injection on my skin? | |
| Even when you press the purple start button, the injection will only happen when the green safety guard is also pushed into the autoinjector. | |
| Can I move the autoinjector around on my skin while I am choosing an injection site? | |
| It is okay to move the autoinjector around on the injection site as long as you do not press the purple start button. However, if you press the purple start button and the green safety guard is pushed into the autoinjector, the injection will begin. | |
| Can I release the purple start button after I start my injection? | |
| You can release the purple start button, but continue to hold the autoinjector firmly against your skin during the injection. | |
| Will the purple start button pop up after I release my thumb? | |
| The purple start button may not pop up after you release your thumb if you held your thumb down during the injection. This is okay. | |
| What do I do if I did not hear a second click? | |
| If you did not hear a second click, you can confirm a complete injection by checking that the window has turned yellow. | |
| Whom do I contact if I need help with the autoinjector or my injection? | |
| A healthcare provider familiar with ENBREL should be able to answer your questions. For more information, call 1-888-4ENBREL (1-888-436-2735) or visit www.enbrel.com. | |
AMGEN®
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
U.S. License Number 1132
©1998 – 2016, 2019-2023 Immunex Corporation. All rights reserved.
<part number> Revised: 06/2023 v18
 This printed material is recyclable.
This printed material is recyclable.
Instructions for Use
Enbrel® (en-brel)
(etanercept)
injection, for subcutaneous use
Single-dose Prefilled Syringe
How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe?
There are 2 types of Enbrel single-dose prefilled syringes:
- The 50 mg/mL single-dose prefilled syringe that contains one 50 mg dose of Enbrel.
- The 25 mg/0.5 mL single-dose prefilled syringe that contains one 25 mg dose of Enbrel.
Your healthcare provider will tell you which one to use.
A 50 mg dose can be given as one injection using a 50 mg/mL single-dose prefilled syringe or as two injections using 25 mg/0.5 mL single-dose prefilled syringes. Your healthcare provider will tell you whether the two injections with 25 mg/0.5 mL single-dose prefilled syringes should be given on the same day once a week or on two different days (3 or 4 days apart) in the same week.
Children must weigh at least 138 pounds to use the Enbrel 50 mg/mL single-dose prefilled syringe. Children who weigh less than 138 pounds should use a different form of Enbrel. The Enbrel 25 mg/0.5 mL single-dose prefilled syringe should not be used in pediatric patients weighing less than 68 pounds.
Important: The Enbrel prefilled syringe is not made with natural rubber latex.
Storage of your Enbrel prefilled syringe
- Store the Enbrel prefilled syringe in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Store the Enbrel prefilled syringe in the original carton to protect from light or physical damage.
- If needed, you may store your Enbrel prefilled syringe at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days.
- Once the Enbrel prefilled syringe has reached room temperature, do not put it back in the refrigerator.
- Throw away any Enbrel prefilled syringe that has been stored at room temperature after 30 days.
- Do not store the Enbrel prefilled syringe in extreme heat or cold. For example, avoid storing Enbrel prefilled syringe in your vehicle's glove box or trunk.
- Do not freeze.
- Do not shake.
- Keep the Enbrel prefilled syringe and all medicines out of the reach of children.
If you have any questions about storage, contact your healthcare provider or call 1-888-4ENBREL (1-888-436-2735) for further instructions.
Step 1: Setting Up for an Injection
- Select a clean, well-lit, flat work surface, such as a table.
- Take the Enbrel carton containing the prefilled syringes out of the refrigerator and place it on your flat work surface. Remove one prefilled syringe and place it on your work surface. Carefully lift the prefilled syringe straight up out of the box. Do not shake the prefilled syringe of Enbrel. Place the carton containing any remaining prefilled syringes back into the refrigerator at 36°F to 46°F (2°C to 8°C).
- Check the expiration date on the prefilled syringe. If the expiration date has passed, do not use the prefilled syringe and contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
- Do not use the prefilled syringe if the needle cover is missing or not securely attached. Call 1-888-4ENBREL (1-888-436-2735).
- For a more comfortable injection, leave the prefilled syringe at room temperature for about 15 to 30 minutes before injecting. Do not remove the needle cover while allowing it to reach room temperature. Do not warm Enbrel in any other way (for example, do not warm it in a microwave or in hot water).
- Hold the prefilled syringe with the covered needle pointing down. If bubbles are seen in the syringe, very gently tap the prefilled syringe to allow any bubbles to rise to the top of the syringe. Turn the syringe so that the purple horizontal lines on the barrel are directly facing you. Check to see if the amount of liquid in the syringe falls between the purple lines. The top of the liquid may be curved. If the syringe does not have the right amount of liquid, do not use that syringe. Contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
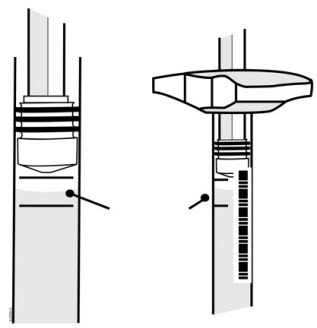
Fill Level Indicator - Assemble the additional supplies you will need for your injection. These include an alcohol swab, a cotton ball or gauze, and a sharps disposal container (see "Step 4: Disposing of Supplies").
- Wash your hands with soap and warm water.
- Make sure the solution in the prefilled syringe is clear and colorless. You may notice small white particles in the solution. These particles are formed from Enbrel and this is acceptable. However, do not inject the solution if it is cloudy or discolored, or contains large or colored particles, call 1-888-4ENBREL (1-888-436-2735).
Step 2: Choosing and Preparing an Injection Site
- Recommended injection sites for Enbrel using a prefilled syringe include:
- the front of the middle thigh
- the stomach area (abdomen), except for the 2-inch area right around the navel (belly button)
- the outer area of the upper arm (only if someone else is giving you the injection)
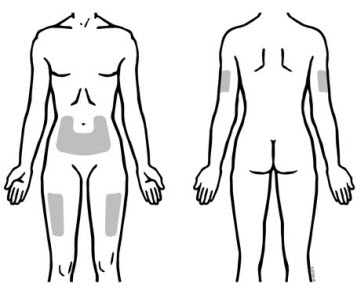
Front Back
- Rotate the site for each injection. Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
- If you have psoriasis, you should not inject directly into any raised, thick, red, or scaly skin patches or lesions.
- To prepare the area of skin where Enbrel is to be injected, wipe the injection site with an alcohol swab. Do not touch this area again before giving the injection.
Step 3: Injecting Enbrel Using a Prefilled Syringe
Do not remove the needle cover from the prefilled syringe until you are ready to inject.
- Pick up the prefilled syringe from your flat work surface. Hold the barrel of the prefilled syringe with one hand and pull the needle cover straight off, only when you are ready to inject. Do not leave the needle cover off for more than 5 minutes. This can dry out the medicine.
To avoid damaging the needle, do not twist or bend the needle cover while you are removing it, and do not try to put the needle cover back onto the prefilled syringe.
When you remove the needle cover, there may be a drop of liquid at the end of the needle; this is normal. Do not touch the needle or allow it to touch any surface. Do not touch or bump the plunger. Doing so could cause the liquid to leak out. - Holding the syringe with the needle pointing up, check the syringe for air bubbles. If there are bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe. Slowly push the plunger up to force the air bubbles out of the syringe.
- Holding the syringe in one hand like a pencil, use the other hand to gently pinch a fold of skin at the cleaned injection site and hold it firmly.
- With a quick and "dart-like" motion, insert the needle at a 45-degree angle into the skin.
- When the needle is completely inserted into the skin, let go of the skin that you are holding. With your free hand, hold the syringe near its base to stabilize it. Then push the plunger to inject all of the Enbrel solution at a slow, steady rate.
- When the syringe is empty, pull the needle out of the skin, being careful to keep it at the same angle as inserted. There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site for 10 seconds. Do not rub the injection site. If needed, you may cover the injection site with a bandage.
The syringe should never be reused. Never recap a needle.
- Put the used prefilled syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) prefilled syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not reuse the syringe.
- Do not recycle the syringe or sharps disposal container or throw them into household trash.
Important: Always keep the sharps disposal container out of the reach of children.
A healthcare provider familiar with Enbrel should answer all questions. Call 1-888-4ENBREL (1-888-436-2735) or visit www.enbrel.com for more information about Enbrel.
This Instructions for Use have been approved by the U.S. Food and Drug Administration.
AMGEN®
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
U.S. License Number 1132
© 1998 – 2016, 2019-2020, 2022-2023 Immunex Corporation. All rights reserved.
1XXXXXX – v12
Revised: 06/2023
 This printed material is recyclable.
This printed material is recyclable.
Instructions for Use
Enbrel® (en-brel)
(etanercept)
for injection, for subcutaneous use
Multiple-dose Vial
How do I prepare and give an injection with Enbrel multiple-dose vial?
A multiple-dose vial contains 25 mg of Enbrel.
Storage of Enbrel multiple-dose vial
- Store Enbrel multiple-dose vial in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Store Enbrel multiple-dose vial in the original carton to protect from light or physical damage.
- If needed, you may store your Enbrel multiple-dose vial and diluent syringe (dose tray) at room temperature between 68°F to 77°F (20°C to 25°C) for up to 14 days.
○ Once Enbrel multiple-dose vial has reached room temperature, do not put it back in the refrigerator.
- Throw away Enbrel multiple-dose vial that has been stored at room temperature after 14 days.
- Mixed (reconstituted) Enbrel multiple-dose vial should be used right away or kept in the refrigerator at 36°F to 46°F (2°C to 8°C) for up to 14 days.
-
Do not store Enbrel multiple-dose vial in extreme heat or cold. For example, avoid storing Enbrel multiple-dose vial in your vehicle’s glove box or trunk.
-
Do not freeze.
-
Do not shake.
- Keep Enbrel multiple-dose vial and all medicines out of the reach of children.
If you have any questions about storage, contact your healthcare provider or call 1-888-4ENBREL (1-888-436-2735) for further instructions.
Step 1: Setting Up for an Injection
- Select a clean, well-lit, flat work surface, such as a table.
- Take the Enbrel dose tray out of the refrigerator and place it on your flat work surface.
- Check the expiration date on the dose tray. If the expiration date has passed, do not use the dose tray. Also check to make sure the dose tray has 5 items as pictured below:
• One prefilled diluent syringe containing 1 mL of diluent (liquid) with attached adapter and twist-off cap
• One plunger
• One Enbrel vial
• One 27-gauge ½ inch needle in hard plastic cover
• One vial adapter
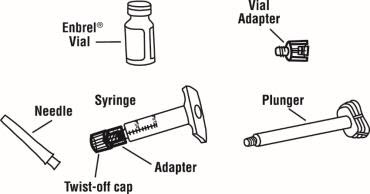
If the expiration date has passed, the five items are not included in the dose tray or if any item looks damaged, contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
- For a more comfortable injection, leave the dose tray at room temperature for about 15 to 30 minutes before injecting.
- Wash your hands with soap and warm water.
- Peel the paper seal off the dose tray and remove all items.
- Inspect the volume of diluent in the syringe with the twist-off cap pointing down. Use the unit markings on the side of the syringe to make sure there is at least 1 mL of liquid in the syringe. If the level of liquid is below the 1 mL mark, do not use. Contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
-
Do not use the syringe if the twist-off cap is missing or not securely attached. Call 1-888-4ENBREL (1-888-436-2735).
- Two alcohol swabs should be available for the preparation and injection of Enbrel. Alcohol swabs can be found at your local drug store.
Step 2: Preparing the Enbrel Solution
There are two methods for preparing the Enbrel solution. For some children, one vial of Enbrel solution can be used for more than one dose. The free-hand method should be used for children on Enbrel who are using one vial of Enbrel solution for more than one dose. You should not use the vial adapter method if you will be using the vial more than once. Ask your healthcare provider if you have questions about which method to use.
- The Vial Adapter Method
Adults and larger children on Enbrel may use the vial adapter device to assist with mixing the powder with the liquid and withdrawing Enbrel, and then use a 27-gauge needle to inject the dose. This method should not be used for children using multiple doses from the same vial of Enbrel. The instructions for using the vial adapter method are in Step 2A.
- The Free-Hand Method
In the free-hand method, a 25-gauge needle is used to assist with mixing the powder with the liquid and withdrawing Enbrel, and a 27-gauge needle is used to inject the dose. Obtain 25-gauge needles from your healthcare provider. Instructions for using the free-hand method are in Step 2B.
The instructions for preparing additional doses from the same vial of Enbrel solution are in Step 3. For each additional dose, you will need two new needles (one 25-gauge needle to withdraw the solution and one 27-gauge needle for injection) and one new empty syringe (1 mL). Never reuse a syringe or needle.
If you are using the vial of Enbrel for more than one dose, you should write the date you mixed the powder and liquid in the area marked “Mixing Date:” on the sticker supplied with these instructions, and attach the sticker to the Enbrel vial.
After you have withdrawn the dose of Enbrel that you need, store the Enbrel vial (in the dose tray) in the refrigerator at 36º to 46ºF (2º to 8ºC) as soon as possible, but always within 4 hours of mixing the solution. Do not freeze. If you have any questions about storage, contact your healthcare provider or call 1-888-4ENBREL (1-888-436-2735) for further instructions.
The Enbrel solution must be used within 14 days of the mixing date. You should discard the Enbrel vial and any remaining solution if it is not used within 14 days. Do not mix any remaining liquid in one vial of Enbrel solution with another.
STEP 2A: Vial Adapter Method
- Remove the pink plastic cap from the Enbrel vial. Do not remove the gray stopper or silver metal ring around the top of the Enbrel vial.
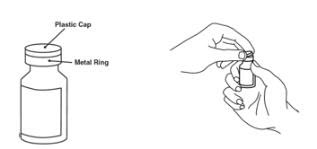
- Place the Enbrel vial on your flat work surface or turn your dose tray upside down and place your Enbrel vial in the round space marked “V”. Use one alcohol swab to clean the gray stopper on the Enbrel vial. Do not touch the gray stopper with your hands.
- Open the wrapper that contains the 27-gauge needle by peeling apart the tabs and set the needle aside for later use.
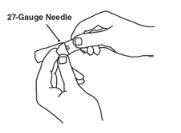
- Open the wrapper that contains the vial adapter by peeling apart the tabs and set the vial adapter aside for later use. Do not touch the vial adapter’s twist-on end or the spike inside.
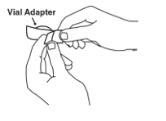
- Slide the plunger into the flange end of the syringe.
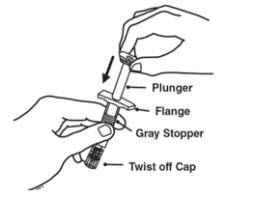
- Attach the plunger to the gray rubber stopper in the syringe by turning the plunger clockwise until you feel a slight resistance.
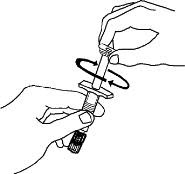
- Remove the twist-off cap from the prefilled diluent syringe by turning counter-clockwise. Do not bump or touch the plunger. Doing so could cause the liquid to leak out. You may see a drop of liquid when removing the cap. This is normal. Place the cap on your flat work surface. Do not touch the syringe tip.
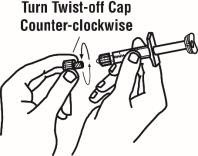
- Once the twist-off cap is removed, pick up the vial adapter with your free-hand. Twist the vial adapter onto the syringe, turning clockwise until you feel a slight resistance. Do not over-tighten.
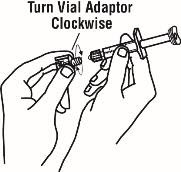
- Hold the Enbrel vial upright on your flat work surface. Grasp the sides of the vial adapter and place it over the top of the Enbrel vial. Do not bump or touch the plunger. Doing so could cause the liquid to leak out. Insert the vial adapter into the gray stopper on the Enbrel vial. The plastic spike inside the vial adapter should puncture the gray stopper. The vial adapter should fit snugly.
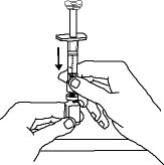
- Hold the Enbrel vial upright on your flat work surface and push the plunger down until all the liquid from the syringe is in the Enbrel vial. You may see foaming (bubbles) in the vial. This is normal.
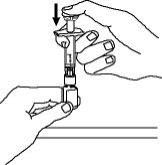
- Gently swirl the Enbrel vial in a circular motion to dissolve the powder. If you used the dose tray to hold your Enbrel vial, take the vial (with the vial adapter and syringe still attached) out of the dose tray, and gently swirl the vial in a circular motion to dissolve the powder.
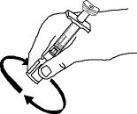
Do not shake. Wait until all the powder dissolves (usually less than 10 minutes). The solution should be clear and colorless. After the powder has completely dissolved, foam (bubbles) may still be present. This is normal. Do not inject the solution if it is discolored, contains lumps, flakes, or particles. If all the powder in the Enbrel vial is not dissolved or there are particles present after 10 minutes, call 1-888-4ENBREL (1-888-436-2735).
- Turn the Enbrel vial upside down. Hold the syringe at eye level and slowly pull the plunger down to the unit markings on the side of the syringe that correspond with your/your child’s dose. For adult patients, remove the entire volume (1 mL), unless otherwise instructed by your healthcare provider. Be careful not to pull the plunger completely out of the syringe. Some white foam may remain in the Enbrel vial. This is normal.
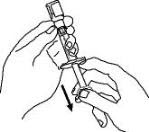
- Check for air bubbles in the syringe. Gently tap the syringe to make any air bubbles rise to the top of the syringe. Slowly push the plunger up to remove the air bubbles. If you push solution back into the vial, slowly pull back on the plunger to again draw the correct amount of solution back into the syringe.
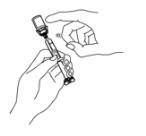
- Remove the syringe from the vial adapter, by holding the vial adapter with one hand and turning the syringe counter-clockwise with your other hand. Do not touch or bump the plunger. Place the Enbrel vial with the vial adapter on your flat work surface.
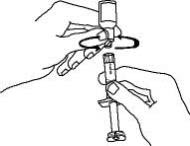
- Continue to hold the barrel of the syringe. With your free-hand, twist the 27-gauge needle onto the tip of the syringe until it fits snugly. Do not remove the needle cover from the syringe. Place the syringe on your flat work surface until you are ready to inject Enbrel.
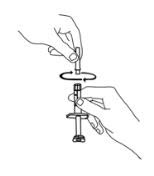
Go to Step 4: Choosing and Preparing an Injection Site.
Step 2B: Free-Hand Method
If you are preparing a dose from an Enbrel vial that was previously used, go to Step 3: Preparing Additional Doses from a Single Enbrel Vial.
- Remove the pink plastic cap from the Enbrel vial. Do not remove the gray stopper or silver metal ring around the top of the Enbrel vial. Write the date you mix the powder and solution on the supplied “Mixing Date:” sticker and attach it to the Enbrel vial.
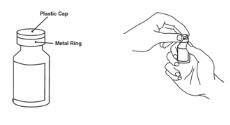
- Place the Enbrel vial on your flat work surface. Use one alcohol swab to clean the gray stopper on the Enbrel vial. Do not touch the gray stopper with your hands.
- Open the wrapper that contains the 25-gauge needle by peeling apart the tabs and set the needle aside for later use. The 25-gauge needle will be used to mix the liquid with the powder and for withdrawing Enbrel from the vial.
- Slide the plunger into the flange end of the syringe.
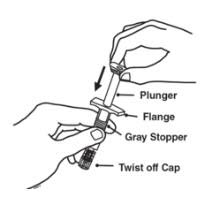
- Attach the plunger to the gray rubber stopper in the syringe by turning the plunger clockwise until you feel a slight resistance.
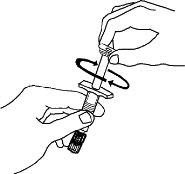
- Remove the twist-off cap from the prefilled diluent syringe by turning counter-clockwise. Do not touch or bump the plunger. Doing so could cause the liquid to leak out. You may see a drop of liquid when removing the cap. This is normal. Place the cap on your flat work surface. Do not touch the syringe tip.
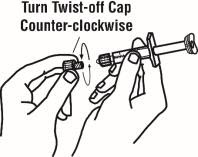
- Continue to hold the barrel of the syringe. With your free-hand, twist the 25-gauge needle onto the tip of the syringe until it fits snugly. Place the syringe on your flat work surface.
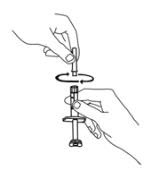
- Open the wrapper that contains the 27-gauge needle by peeling apart the tabs and set the needle aside for later use. The 27-gauge needle will be used to inject the dose.
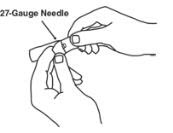
- Pick up the syringe from your flat work surface. Hold the barrel of the syringe with one hand, and pull the needle cover straight off. To avoid damaging the needle, do not twist or bend the needle cover while you are removing it. Do not touch the needle or allow it to touch any surface. Do not touch or bump the plunger. Doing so could cause the liquid to leak out.
- Place the needle cover (open side up) in the round space marked “N” in the Enbrel dose tray.
- Place the Enbrel vial on your flat work surface. Hold the syringe with the needle facing up, and gently pull back on the plunger to pull a small amount of air into the syringe. Then, insert the needle straight down through the center ring of the gray stopper (see illustrations). You should feel a slight resistance and then a “pop” as the needle goes through the center of the stopper. Look for the needle tip inside the open stopper window. If the needle is not correctly lined up with the center of the stopper, you will feel constant resistance as it goes through the stopper and no “pop”. The needle may enter at an angle and bend, break or prevent you from adding diluent into the Enbrel vial.
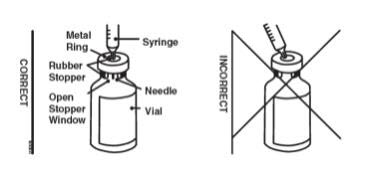
- Push the plunger down very slowly until all liquid from the syringe is in the Enbrel vial. Adding the liquid too fast will cause foaming (bubbles).
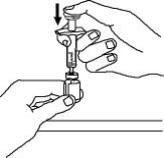
- Leave the syringe in place. Gently swirl the Enbrel vial in a circular motion to dissolve the powder.
Do not shake. Wait until all the powder dissolves (usually less than 10 minutes). The solution should be clear and colorless. After the powder has completely dissolved, foam (bubbles) may still be present. This is normal. Do not inject the solution if it is discolored, contains lumps, flakes, or particles. If all the powder in the Enbrel vial is not dissolved or there are particles present after 10 minutes, call 1-888-4ENBREL (1-888-436-2735).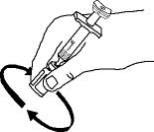
- With the needle in the Enbrel vial, turn the vial upside down. Hold the syringe at eye level and slowly pull the plunger down to the unit markings on the side of the syringe that correspond with the correct dose. Make sure to keep the tip of the needle in the solution. Some white foam may remain in the Enbrel vial. This is normal.
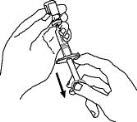
- With the needle still inserted in the Enbrel vial, check for air bubbles in the syringe. Gently tap the syringe to make any air bubbles rise to the top of the syringe. Slowly push the plunger up to remove the air bubbles. If you push solution back into the vial, slowly pull back on the plunger to draw the correct amount of solution back into the syringe.
- Remove the syringe and needle from the Enbrel vial. Keep the needle attached to the syringe and insert the 25-gauge needle straight down into the needle cover in the Enbrel dose tray.
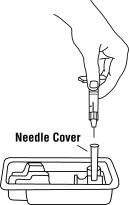
You should hear a “snap” when the needle is secure in the needle cover. Once the needle is secure in the needle cover, untwist the 25-gauge needle from the syringe and dispose of the needle in your sharps disposal container (see “Step 6: Disposing of Supplies”).
- Twist the 27-gauge needle onto the syringe until it fits snugly. Do not remove the needle cover from the syringe. Place the syringe on your flat work surface until you are ready to inject Enbrel.
- If there is enough solution left in the Enbrel vial for another dose, write the date you mixed the powder and liquid in the area marked “Mixing Date:” on the sticker supplied with these instructions, and attach the sticker to the Enbrel vial. Refrigerate the reconstituted (mixed) Enbrel vial (in the dose tray) after mixing. Prepare additional doses from the Enbrel vial as described in Step 3. Otherwise, throw away (discard) the Enbrel vial and any remaining solution.
Go to Step 4: Choosing and Preparing an Injection Site.
Step 3: Preparing Additional Doses from a Single Enbrel Vial
- Select a clean, well-lit, flat work surface, such as a table.
-
Do not reuse the needles and syringes supplied with Enbrel dose tray. You will need new needles and syringes for each additional dose. Your healthcare provider will tell you what type of syringes (1 mL) and needles (25-gauge and 27-gauge) to use. Place the sterile syringe with a 25-gauge needle (for withdrawing Enbrel), a 27-gauge needle (for injecting Enbrel) and two alcohol swabs on your flat work surface.
- Take the vial of Enbrel solution that is stored in the dose tray out of the refrigerator and place it on your flat work surface.
- Check the mixing date you wrote on the sticker on the Enbrel vial. Discard the Enbrel vial if more than 14 days have passed since the Enbrel solution was mixed.
- Wash your hands with soap and warm water.
- Use one alcohol swab to clean the gray stopper on the Enbrel vial. Do not touch the stopper with your hands.
- If the syringe and the 25-gauge needle are not pre-assembled, assemble them as instructed by your healthcare provider.
- Open the wrapper that contains the 27-gauge needle by peeling apart the tabs and set the needle aside for later use. The 27-gauge needle will be used to inject the dose of Enbrel.
- Hold the syringe and pull the needle cover straight off. To avoid damaging the needle, do not twist or bend the needle cover while you are removing it. Do not touch the needle or allow it to touch any surface. Place the needle cover (open side up) in the round space marked “N” in the Enbrel dose tray.
- Place the Enbrel vial on your flat work surface. Hold the syringe with the needle facing up, and gently pull back the plunger to pull a small amount of air into the syringe. Then, insert the 25-gauge needle straight down through the center ring of the gray stopper. You should feel a slight resistance and then a “pop” as the needle goes through the center of the stopper. Look for the needle tip inside the open stopper window. If the needle is not correctly lined up with the center of the stopper, you will feel constant resistance as it goes through the stopper and no “pop”. The needle may enter at an angle and bend, break, or prevent proper withdrawal of Enbrel solution from the vial.
- Keep the needle in the Enbrel vial and turn the vial upside down. Hold the syringe at eye level, and slowly pull the plunger down to the unit markings on the syringe that correspond to your child’s dose. As the amount of solution in the Enbrel vial drops, you may need to pull the needle back just enough to keep the tip of the needle in the solution.
- With the needle still inserted in the Enbrel vial, check for air bubbles in the syringe. Gently tap the syringe to make any air bubbles rise to the top of the syringe. Slowly push the plunger up to remove the air bubbles. If you push solution back into the Enbrel vial, slowly pull back on the plunger to again draw the correct amount of solution back into the syringe.
- Remove the syringe and needle from the Enbrel vial. Keep the needle attached to the syringe and insert the 25-gauge needle straight down into the needle cover in the Enbrel dose tray. You should hear a “snap” when the needle is secure in the needle cover. Once the needle is secure in the needle cover, remove the 25-gauge needle from the syringe and dispose of the needle in a sharps disposal container (see “Step 6: Disposing of Supplies”).
- Attach the 27-gauge needle onto the tip of the syringe until it fits snugly. Do not remove the needle cover from the syringe. Place the syringe on your flat work surface until you are ready to inject Enbrel.
Step 4: Choosing and Preparing an Injection Site
- The 3 recommended injection sites for Enbrel include:
• the front of the middle thighs
• the stomach area (abdomen), except for the 2-inch area right around the navel (belly button)
• the outer area of the upper arms (only if someone else is giving you the injection)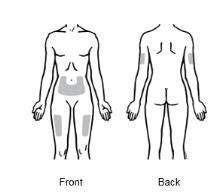
- Rotate the site for each injection. Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
- If you have psoriasis, you should not inject directly into any raised, thick, red, or scaly skin patches or lesions.
- To prepare the area of skin where Enbrel is to be injected, wipe the injection site with a new alcohol swab. Do not touch this area again before giving the injection.
Step 5: Injecting the Enbrel Solution
Do not remove the needle cover from the syringe until you are ready to inject.
- Pick up the syringe from your flat work surface. Hold the barrel of the syringe with one hand and pull the needle cover straight off. To avoid damaging the needle, do not twist or bend the needle cover while you are removing it, and do not try to put the needle cover back onto the syringe. When you remove the needle cover, there may be a drop of liquid at the end of the needle. This is normal. Do not touch the needle or allow it to touch any surface. Do not touch or bump the plunger. Doing so could cause the liquid to leak out.
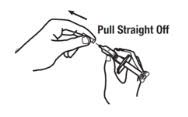
- With one hand, gently pinch the cleaned area of skin and hold it firmly. With the other hand, hold the syringe (like a pencil) at a 45-degree angle to the skin.
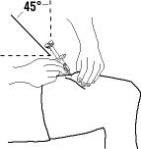
- With a quick and "dart-like" motion, insert the needle at a 45-degree angle into the skin.
- When the needle is completely inserted into the skin, let go of the skin that you are holding. With your free-hand, hold the syringe near its base to stabilize it. Then push the plunger to inject all of the Enbrel solution at a slow, steady rate.
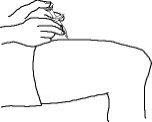
- When the syringe is empty, pull the needle out of the skin, being careful to keep it at the same angle as inserted.
- There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site for 10 seconds. Do not rub the injection site. If needed, you may cover the injection site with a bandage.
- If your healthcare provider has instructed you to take two Enbrel injections on the same day, repeat the steps to prepare and give an injection of Enbrel. Choose and prepare a new injection site for the second injection.
- For the Free-Hand Method: If there is enough solution left in the Enbrel vial for another dose, refrigerate the reconstituted (mixed) Enbrel vial (in the dose tray) after use. Otherwise, discard the Enbrel vial and any remaining solution.
The syringe, needles, and vial adapter should never be reused. Never recap a needle.
- Put the used syringes, needles, and vials in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the needles, syringes, and vials in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
○ made of a heavy-duty plastic,
○ can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
○ upright and stable during use,
○ leak-resistant, and
○ properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
-
Do not reuse the syringe or vial.
- Do not recycle the syringe, vial, or sharps disposal container or throw them into household trash.
Important: Always keep the sharps disposal container out of the reach of children.
A healthcare provider familiar with Enbrel should answer all questions. Call 1-888-4ENBREL (1-888-436-2735) or visit www.enbrel.com for more information about Enbrel.
This Instructions for Use have been approved by the U.S. Food and Drug Administration.
AMGEN®
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
U.S. License Number 1132
© 1998 – 2016, 2019 Immunex Corporation. All rights reserved.
1XXXXXX – v9
Revised: 10/2019
 This printed material is recyclable.
This printed material is recyclable.
Front Panel Text
Read these instructions before using an AutoTouch® reusable autoinjector for use with Enbrel Mini® (etanercept) single-dose prefilled cartridge.
Side 1 Text
Your healthcare provider has prescribed Enbrel® AutoTouch reusable autoinjector for your injections. If your healthcare provider decides that you or a caregiver may be able to give your injections of Enbrel at home, you should receive training on the right way to prepare and inject Enbrel. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider. Call your healthcare provider if you or your caregiver has any questions about the right way to inject Enbrel.
Read these instructions before using an AutoTouch reusable autoinjector for use with Enbrel Mini (etanercept) single-dose prefilled cartridge.
| Speed switch Changing injection speed The AutoTouch reusable autoinjector allows you to choose the speed of your injection. You can try the three speeds to decide which speed is most comfortable for you.
| 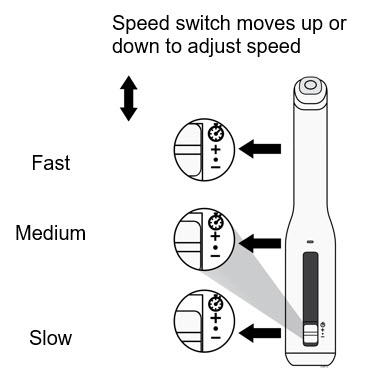 |
| Sound switch Turning sounds off The AutoTouch autoinjector makes sounds (
|  |
| Turn over for step-by-step INSTRUCTIONS FOR USE and other information. |
Side 2 Text
INSTRUCTIONS FOR USE
ENBREL® (en-brel)
(etanercept)
injection, for subcutaneous use
Enbrel Mini® Single-dose Prefilled Cartridge
for use with all AutoTouch® Reusable Autoinjectors
| Guide to parts | |
| AutoTouch reusable autoinjector | |
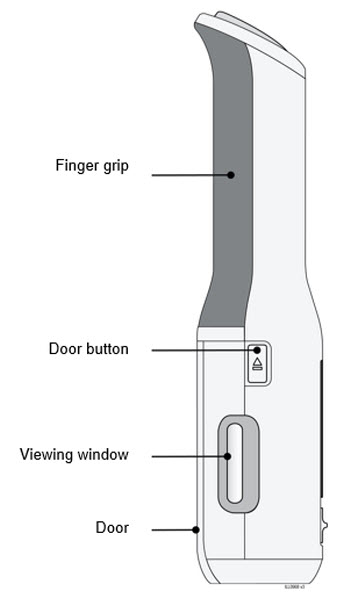 |  |
| Enbrel Mini® single-dose cartridge | |
|
Before use
|
After use
|
Important: The needle is inside Enbrel Mini.
Important
The AutoTouch® reusable autoinjector is used with the Enbrel Mini® single-dose prefilled cartridge to administer your dose of Enbrel®. The Enbrel Mini single-dose prefilled cartridge contains one 50 mg single-dose of Enbrel.
Before you use the AutoTouch reusable autoinjector with Enbrel Mini single-dose prefilled cartridge for use with Enbrel, read this important information:
Storing your Enbrel Mini single-dose cartridges:
- Store Enbrel Mini, which contains Enbrel, in the refrigerator between 36°F to 46°F (2°C to 8°C).
- If needed, you may store Enbrel Mini at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days.
- Once Enbrel has reached room temperature, do not put it back in the refrigerator.
- Throw away (dispose of) Enbrel Mini that has been stored at room temperature after 30 days.
- Do not store Enbrel Mini in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
- Do not freeze.
- Do not shake.
- Store Enbrel Mini in the original carton to protect from light or damage.
Using your Enbrel Mini single-dose cartridges:
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- Do not use Enbrel Mini after the expiration date printed on the label.
- Do not shake Enbrel Mini.
- Do not remove the purple cap from Enbrel Mini until it is inside the AutoTouch reusable autoinjector and you are ready to inject.
- Do not use Enbrel Mini if it has been dropped on a hard surface. Part of Enbrel Mini may be broken even if you cannot see the break. Use a new Enbrel Mini cartridge, and call 1-888-4Enbrel (1-888-436-2735).
- The AutoTouch reusable autoinjector and the Enbrel Mini cartridge are not made with natural rubber latex.
- Children must weigh at least 138 pounds to receive 50 mg Enbrel Mini. Children who weigh less than 138 pounds should receive a different form of Enbrel.
Storing your AutoTouch reusable autoinjector:
- Store AutoTouch at room temperature in a dry, safe place, such as a cabinet or drawer at 50°F to 104°F (10°C to 40°C).
- Use an alcohol wipe to clean the bottom (injection end) before and after each use.
- Do not store AutoTouch in the refrigerator with Enbrel Mini.
Keep Enbrel Mini and AutoTouch and all medicines out of the reach of children.
For more information or help, visit www.enbrel.com or call 1-888-4Enbrel (1-888-436-2735). Read your AutoTouch User Manual for additional care and storage information.
Step 1: Prepare
A Remove one Enbrel Mini® single-dose cartridge from the carton.
- Put the carton containing any unused Enbrel Mini back in the refrigerator.
- Leave Enbrel Mini at room temperature for at least 30 minutes before injecting.
- Do not put Enbrel Mini back in the refrigerator once it has reached room temperature.
- Do not try to warm Enbrel Mini by using a heat source such as hot water or microwave.
- Do not shake Enbrel Mini.
- Do not remove the purple cap from Enbrel Mini yet.
B Inspect the Enbrel Mini single-dose prefilled cartridge.
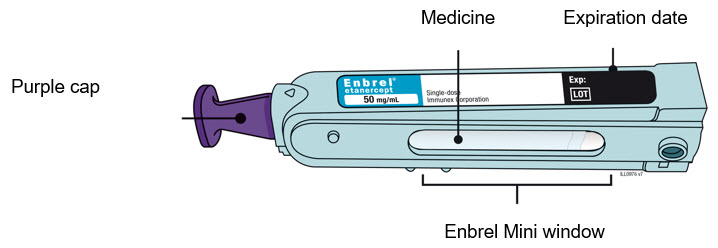
Make sure the medicine in the window is clear and colorless. It is okay if you see small white particles in the medicine window.
- Do not use Enbrel Mini if the medicine is cloudy, discolored, or contains large lumps, flakes, or colored particles.
- Do not use Enbrel Mini if any part appears cracked or broken.
- Do not use Enbrel Mini if the purple cap is missing or not securely attached.
- Do not use Enbrel Mini if the expiration date printed on the label has passed.
In all cases, use a new Enbrel Mini, and call 1-888-4Enbrel (1-888-436-2735).
C Gather all materials you need for your injection.
Wash your hands thoroughly with soap and water.
Place the following on a clean, well-lit, flat surface:
- AutoTouch®
- 1 Enbrel Mini®
- 3 alcohol wipes
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container
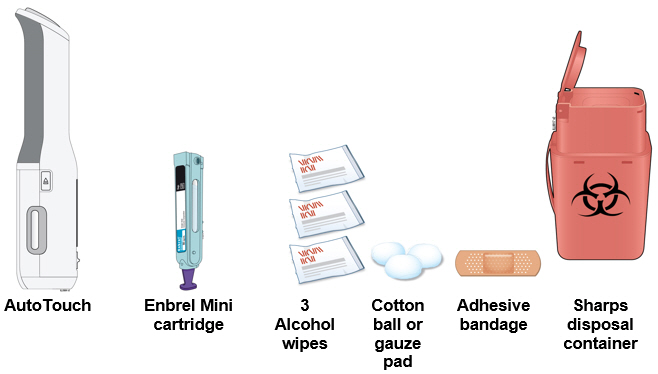
D Prepare and clean your injection site.
Also clean the injection end of the AutoTouch® with a new alcohol wipe.
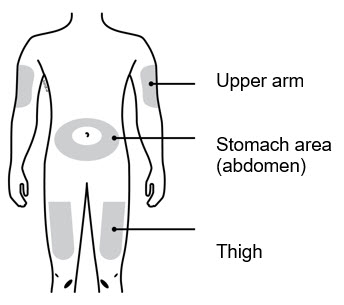 |  |
Clean your injection site with an alcohol wipe. Let your skin dry.
You can use:
- Your thigh
- Stomach area (abdomen), except for a 2-inch area right around your navel
- Outer area of upper arm (only if someone else is giving you the injection)
Important:
- Do not touch this area again before injecting.
- Choose a different site each time you give yourself an injection. If you want to use the same injection site, make sure it is not the same spot on the injection site you used for a previous injection.
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
- If you have psoriasis, you should avoid injecting directly into raised, thick, red, or scaly skin patches or lesions.
Step 2: Get ready
E Press the door button to open the door.
F Hold the Enbrel Mini® single-dose cartridge with the labeled side facing out and slide into door.
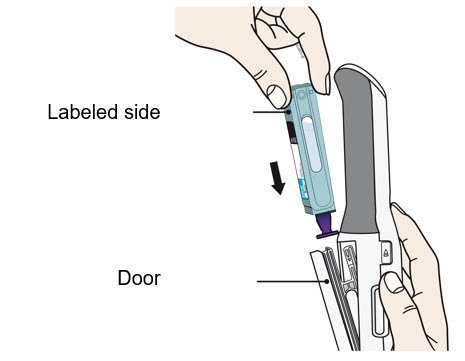
Hold with the labeled side facing out and the purple cap pointing down. Then slide Enbrel Mini into the door. It will slide all the way down into AutoTouch®.
- Do not force Enbrel Mini into AutoTouch if it does not slide in easily. Check that it is positioned correctly inside AutoTouch.
G Close the door.
|
|
|
| When you see the viewing window light up and hear a "chime", this means that Enbrel Mini® has been loaded properly. AutoTouch is now in the awake mode. Note: If the sound switch is set to the "sounds off" position, you will not hear this sound. To turn the "sounds on", slide the sound switch down. The red bar will no longer be visible. | Sounds on Slide switch down Slide switch down | Sounds off
 Slide switch up Slide switch up |
Important: Do not remove the purple cap until you are ready to give your injection.
Step 3: Inject
 | Read the following information before proceeding with your injection. |
You will now be performing the following tasks in order:
- Hold AutoTouch® correctly.
- Remove the purple cap.
- Confirm that AutoTouch is "awake".
- Place and hold AutoTouch on the injection site.
- After the status button turns green, press the status button to start the injection.
- Wait for the injection to finish (ensure that AutoTouch maintains full contact with the skin).
- Confirm the injection is complete.
Do not give your injection until you have read these instructions and feel comfortable with how to use AutoTouch the right way.
H Hold the AutoTouch reusable autoinjector with fingers wrapped around the gray finger grip as shown.
| 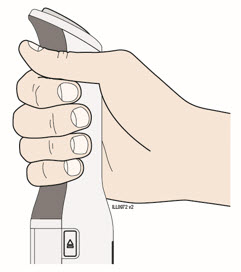 |
I When you are ready to inject, pull the purple cap straight down and off. Do not leave the purple cap off for more than 5 minutes. This can dry out the medicine.
Dispose of the purple cap in a sharps disposal container. Small parts such as the purple cap may cause a choking hazard for children and pets.
| 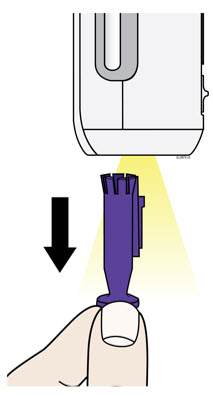 |
J Place and hold on the skin. Wait for the status button to turn green and a chime to sound.
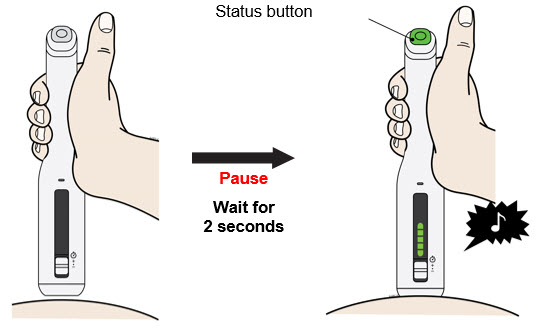
Gently place and hold the AutoTouch® reusable autoinjector on your skin and wait 2 seconds for the status button to turn green. You will hear a "chime" (unless the sound switch is set to the "sounds off" position).
- Keep your thumb off the status button during this step.
- Do not press the autoinjector hard on your skin. There is no need to stretch or pinch your skin.
K Press and release the status button. The status button will start to flash green. You will hear a click when the injection starts.
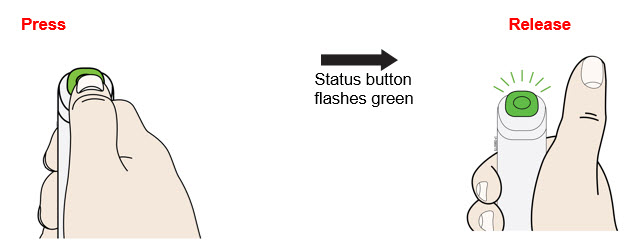
The green status button means your injection has started. You will hear a motor noise and the status button will flash green.
Important: Do not lift AutoTouch® off your skin while the injection is in progress. Continue to hold AutoTouch straight and steady.
L The injection is finished when the green light turns off and a "chime" sounds. Check to make sure you do not see a red status button.
 |  |  |
| (light off) Injection finished | (red light on) Injection error Call 1-888-4Enbrel (1-888-436-2735) |
Lift AutoTouch® off your skin.
When your injection is finished, you will hear a motor noise for a few seconds. When finished, the door will automatically open. Do not block the door with your hand.
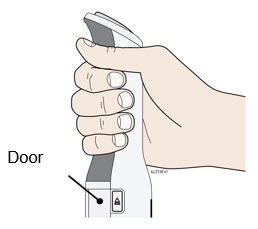
Important: When you remove AutoTouch, if the status button has turned red, call 1-888-4Enbrel (1-888-436-2735). If it looks like the medicine is still injecting or if you see medicine in the Enbrel Mini® window, this means you may not have received a full dose. Call your healthcare provider right away.
Step 4: Finish
M Remove and throw away (dispose of) the Enbrel Mini® single-dose prefilled cartridge.
When the door opens, remove Enbrel Mini, close the door, then put it in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) Enbrel Mini in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant, and properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not reuse Enbrel Mini.
- Do not recycle Enbrel Mini or the sharps disposal container or throw them into household trash.
Important: Always keep the sharps disposal container out of the reach of children.
N Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage, if needed.
O Clean and store the AutoTouch® reusable autoinjector.
Clean the injection end of the autoinjector with a new alcohol wipe and allow to dry.
Store AutoTouch at room temperature in a dry, safe place such as in a cabinet or drawer.
Important: Do not store AutoTouch in the refrigerator.
| Why is the status button flashing red? | ||
 | Low battery: The battery is very low. Only a few injections remain. Note: battery icon may be yellow on some devices. Call 1-888-4Enbrel (1-888-436-2735) for a replacement AutoTouch reusable autoinjector. | 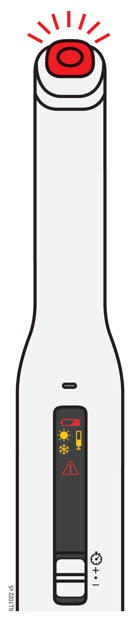 |
 | Battery dead: The battery is dead. Note: battery icon may be yellow on some devices. Call 1-888-4Enbrel (1-888-436-2735) for a replacement AutoTouch reusable autoinjector. | |
 | Too hot or too cold: AutoTouch reusable autoinjector is too hot, or too cold. Allow to naturally cool or warm to room temperature until symbols are gone. | |
 | Enbrel Mini® problem: There are several reasons you might get this error. See the User Manual for more information or call 1-888-4Enbrel (1-888-436-2735). | |
 | Needle exposure: The needle may be exposed and an incomplete dose may have been injected. Look for medicine in Enbrel Mini. Call your healthcare provider if you feel you have given yourself an incomplete injection. Take special care when removing and handling Enbrel Mini by disposing properly in the sharps container. | |
 | AutoTouch reusable autoinjector failure: Call 1-888-4Enbrel (1-888-436-2735) for reset instructions or a replacement AutoTouch reusable autoinjector. | |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMGEN®
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
©1998 – 2018, 2021-2023 Immunex Corporation. All rights reserved.
[partnumber]
Revised: 06/2023 v7
U.S. License Number 1132
 This printed material is recyclable.
This printed material is recyclable.
AutoTouch® reusable
autoinjector for use with
Enbrel Mini® (etanercept)
single-dose prefilled cartridge
User Manual
| Table of Contents | |
| Getting Started | 4 |
| Reference Guide | 5 |
| Resources | 6 |
| Guide to Parts | 8 |
| Troubleshooting: Error Symbols | 18 |
| Troubleshooting: Common Problems | 24 |
| Storage & Handling | 28 |
| Cleaning Instructions | 30 |
| Warnings | 31 |
| Technical Information | 32 |
| Symbol Table | 34 |
| Getting Started |
This User Manual contains helpful information about your AutoTouch® reusable autoinjector. It includes resources, care details, and a troubleshooting guide to save for ongoing reference.
To learn how to inject using AutoTouch, see your healthcare provider for injection training and use the Instructions for Use included in the carton.
Instructions for Use
A fold-out, step-by-step Instructions for Use is provided in the carton, which provides you with full instructions to learn how to give yourself a safe, successful and accurate injection.
Note: Please read the full Instructions for Use included in the carton.
The Reference Guide on the next page should only be used when you have successfully completed an injection using the Instructions for Use.
| Reference Guide |
| Prepare and clean your injection site. | Hold the Enbrel Mini® single-dose prefilled cartridge with labeled side facing out and slide into door. Close door. Remove purple cap. | ||||
| 1 |
| 2 |
|
|
|
| Place and hold on skin. Wait for the status button to turn green. | To start injection: Press and release the green status button. | Injection is finished when you hear a chime and all lights are turned off. | |||
| 3 |
| 4 |
| 5 |
|
| Resources |
Personalized help from ENBREL Support™
Here are just a few of the benefits available at no cost to you:
- Live nurses online or on the phone seven days a week, 8 AM to 11 PM (ET)
- ENBREL Support copay card and other financial help
- Medication and refill reminders by phone or email
- Needle disposal containers through the Sharps Mail-Back Program
Sign up at 1-888-4ENBREL (1-888-436-2735) or EnbrelSupport.com.
| Guide to Parts |
AutoTouch® reusable autoinjector
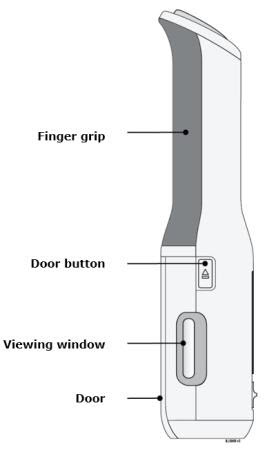
| Finger grip
When injecting, hold the AutoTouch® reusable autoinjector with fingers wrapped around the gray finger grip. |
|
Door button
Press this button to open the door for insertion of the Enbrel Mini® single-dose prefilled cartridge. When your injection is finished, the door will open automatically.
Viewing window
During an injection, you can look through this window and see the plunger lowering to deliver your medicine. When AutoTouch is awake, the viewing window will light up. If Enbrel Mini is in AutoTouch, and the viewing window has no light, then press the status button to wake up AutoTouch.
You will open this door and insert Enbrel Mini into AutoTouch. The door will open automatically when your injection is finished. When inserted properly, Enbrel Mini will slide freely and completely into and out of the door.
| Guide to Parts |
AutoTouch® reusable autoinjector
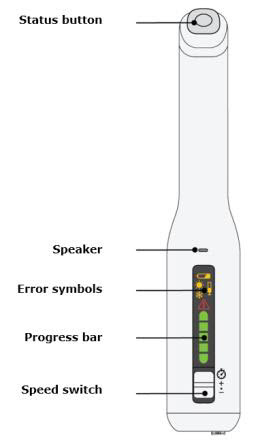
Status button
Press this button to begin an injection. Lights will indicate the status of your injection.
Green:
Ready to press and start injection.
Blinking green:
The needle is inserting and your injection is in progress.
Red:
An error has occurred. See the Troubleshooting section of this manual.
Progress bar
These stacked green bars are fully lit when your injection starts, and the bar of lights decrease as your injection is in progress. The lights will disappear when your injection is finished.
Speed switch
The AutoTouch® reusable autoinjector allows you to choose from three injection speeds. The pre-set speed is medium.
(+) is faster
(•) is medium
(–) is slower
| Guide to Parts |
AutoTouch® reusable autoinjector
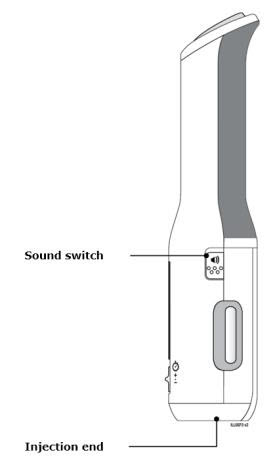
Sound switch
Sounds can be turned off and on. (Sounds on) Slide switch down. (Sounds off) Slide switch up so that the red bar is visible.
Note: Error sounds will still be heard if the sounds have been turned off.
Injection end
The entire injection end must be touching your skin during injections. This is also where the needle will come out.
Skin sensor
A skin sensor is located on the injection end. When the injection end is placed on skin the start button light will turn green. Hold the injection end flat and steady on your skin throughout the entire injection process.
Site light
When the Enbrel Mini® single-dose prefilled cartridge is loaded and the purple cap is removed, the injection end will light up to help you see your injection site.
| Guide to Parts |
Enbrel Mini® single-dose prefilled cartridge
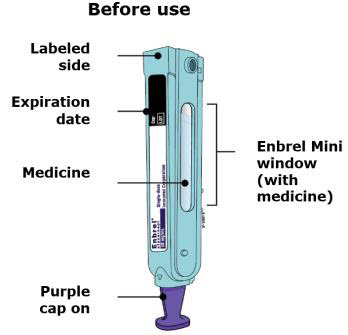
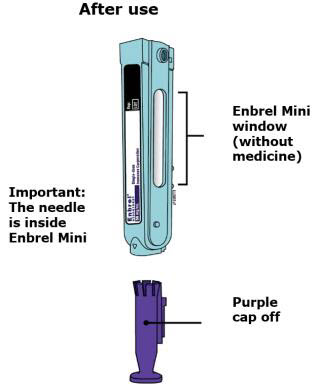
Labeled side
When inserting the Enbrel Mini® cartridge make certain that the labeled side is facing out. Then slide Enbrel Mini into the door. It will slide all the way down into the AutoTouch® reusable autoinjector.
Expiration date
Confirm expiration date printed on the label has not passed.
Enbrel Mini window
This is where you will look to examine your medicine before starting an injection. See Instructions for Use for more information.
Purple cap
The purple cap keeps your medicine safe. Do not remove the cap on Enbrel Mini before loading it into AutoTouch. Load Enbrel Mini with the purple cap on, and remove the cap only when you are ready to give yourself the injection. Do not leave the purple cap off for more than five minutes. This can dry out the medicine.
See the Instructions for Use for more detail about loading Enbrel Mini and when to remove the purple cap.
| Guide to Parts |
AutoTouch® reusable autoinjector
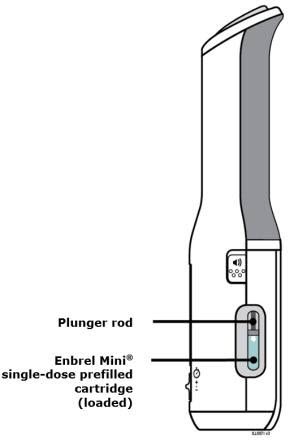
How it works.
When you push the status button to start an injection, the AutoTouch® reusable autoinjector pushes a hidden needle out of the Enbrel Mini® single-dose prefilled cartridge into your skin. Then, a plunger rod will push into Enbrel Mini, injecting medicine into your body. After the medicine is injected, the rod will pull back up and the needle will withdraw from your skin. After a successful injection all lights will turn off and the door will open. The needle stays hidden at all times during the injection process.
What you will hear.
Needle insertion and plunger rod are operated by motors. You will hear a motor noise as the rod moves down and then back up again. Whenever you hear a motorized noise, you will know that an injection is in progress.
What you will see.
During the injection, the viewing window light will be on, and the plunger rod will move through the window. Also during the injection, the status light will be flashing green, and the progress bar will decrease. After a successful injection, the progress bar, and status button will turn off, and AutoTouch will open the door.
If the status light turns red, and beeps for more than a few seconds, an error has occurred. Use the troubleshooting section of this manual to see what to do if this happens.
| Troubleshooting: Error Symbols |
Error Symbols
The AutoTouch® reusable autoinjector makes a chime sound, lights the status button red, and displays an error symbol if there is a problem.
See the following description of each error symbol, possible reasons for the error, and actions you can take.
 Low battery
Low battery
Symbol: Orange battery and error sound.
Problem: The battery is running very low. Less than three weeks or three injections remain. The battery has a life of two years or 130 injections from your first injection. The battery is not replaceable or rechargeable.
Action: Call 1-888-4ENBREL (1-888-436-2735) for a replacement AutoTouch reusable autoinjector.
 Battery Dead
Battery Dead
Symbol: Orange battery and red triangle with exclamation point, red status button, and error sound.
Problem: The AutoTouch® reusable autoinjector battery is dead.
Action: Call the 1-888-4ENBREL (1-888-436-2735) for a replacement AutoTouch reusable autoinjector.
 Too hot or cold
Too hot or cold
Symbol: Orange sun and a snowflake, and an error sound.
Problem: AutoTouch is too hot or too cold. This will lock the door.
Action: Place AutoTouch at room temperature in a safe, dry place and allow it to naturally cool or warm to room temperature. Then try again. Store the AutoTouch reusable autoinjector in a dry, safe place, such as a cabinet or drawer at 50° F to 104° F (10° C to 40° C).
Troubleshooting: Error Symbols
 Enbrel Mini problem
Enbrel Mini problem
Symbol: Orange Enbrel Mini® single-dose prefilled cartridge with blinking red status button and error alert sound.
Problem: There are five possible reasons you are getting this error. Please read below.
Problem #1: The purple cap was removed before inserting Enbrel Mini.
Action: Wait for the error to clear and close the door without Enbrel Mini in, and try again with a new Enbrel Mini. Make sure to leave the purple cap on until Enbrel Mini has been placed inside the AutoTouch® reusable autoinjector and the door is closed. Call 1-888-4ENBREL (1-888-436-2735) for a replacement Enbrel Mini.
Problem #2: Sensor lost skin contact during injection.
Action: Hold the injection end of AutoTouch on your skin throughout the entire injection until the green status button turns off, even if the motor sounds stop. Lifting or shifting on your skin during injection may lead to an incomplete injection. Call your healthcare provider if you feel you have given yourself an incomplete injection.
Problem #3: Enbrel Mini® single-dose prefilled cartridge is defective or not recognized as an Amgen product.
Action: Remove Enbrel Mini. Replace with a new Enbrel Mini. Call 1-888-4ENBREL (1-888-436-2735) for a replacement Enbrel Mini.
Problem #4: Door is held closed for more than one minute.
Action: Remove your hand from the door. When the door opens, remove Enbrel Mini from AutoTouch®, if present. Wait for the error symbol to stop blinking and close the door. If you have not given yourself an injection, place a new Enbrel Mini in AutoTouch and continue. If the error symbol remains on, call 1-888-4ENBREL (1-888-436-2735).
Problem #5: AutoTouch has been dropped.
Action: A dropped AutoTouch is not safe to use. Call 1-888-4ENBREL (1-888-436-2735) for a replacement.
Troubleshooting: Error Symbols
 Needle Exposure
Needle Exposure
Symbol: Orange Enbrel Mini® single-dose prefilled cartridge, a red triangle with an exclamation point, red status button, and error sound.
Problem: A problem occurred during an injection and the needle may be exposed.
Action: If there is still fluid in Enbrel Mini, an incomplete dose may have been injected. Call your healthcare provider if you feel you have given yourself an incomplete injection. Call 1-888-4ENBREL (1-888-436-2735) for further assistance with your AutoTouch®.
Use caution when both Enbrel Mini problem symbol and the AutoTouch® reusable autoinjector failure symbols are lit as the needle may be exposed. Take special care when removing and handling Enbrel Mini. Remove Enbrel Mini then put it in a FDA-cleared sharps disposal container.
 AutoTouch® Reusable Autoinjector Failure
AutoTouch® Reusable Autoinjector Failure
Symbol: Red triangle with an exclamation point, red status button, and error sound.
Problem: Several errors have occurred or AutoTouch has stopped working.
Action: Reset AutoTouch.
To reset AutoTouch:
Hold AutoTouch away from skin and press the status button to wake AutoTouch. The failure symbol should begin blinking and a chime should sound.
While the failure symbol is blinking, press and hold door button until all symbols are temporarily displayed and the status button blinks green. The door button should be held for at least 10 seconds. After a successful reset, if an Enbrel Mini® single-dose prefilled cartridge is still inside AutoTouch, remove it. Close the AutoTouch door. Then, for the next injection, start by pressing the door button to open Enbrel Mini door.
If AutoTouch does not respond after three attempts to reset, call 1-888-4ENBREL (1-888-436-2735).
| Troubleshooting: Common Problems |
Common Problems
Enbrel Mini® single-dose prefilled cartridge is difficult to insert into the door.
Never force Enbrel Mini into the door. When positioned correctly, it will fall freely and completely into and out of the door. If it is difficult to load, double check that you are holding Enbrel Mini as shown.
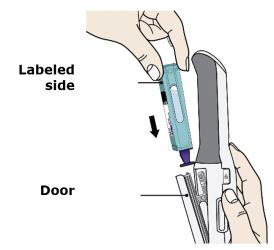
- Purple cap is facing down
- Label facing outwards (away from the handle)
Injection does not start when pressing the status button.
Reason #1: Place the AutoTouch® reusable autoinjector on your skin and wait for the status button to turn green. A skin sensor is located on the injection end. You cannot start an injection unless the injection end of AutoTouch is touching your skin. Hold the injection end on your skin throughout the entire injection.
Reason #2: AutoTouch may be asleep. To conserve battery power, AutoTouch goes into a “sleep mode” after three minutes of no activity. If it seems unresponsive/asleep, remove AutoTouch from your skin and press the status button to wake it up.
| An Enbrel Mini error symbol appears immediately after loading the Enbrel Mini® single-dose prefilled cartridge.
Reason: This will happen if the purple cap has been removed before inserting Enbrel Mini into the door. Do not reuse or recap. Begin again using a new Enbrel Mini. Do not remove the purple cap until after Enbrel Mini has been inserted into AutoTouch. |
| Troubleshooting: Common Problems |
| Injection aborts or an error symbol appears while the injection is in process.
The AutoTouch® reusable autoinjector will abort an injection if the sensor loses skin contact. Avoid adjusting or moving AutoTouch during an injection. Hold AutoTouch on your skin until the green status light turns off and the injection is finished. Shifting, repositioning or lifting from your skin during injection may lead to an incomplete dose. |
| AutoTouch door will not remain closed.
Reason #1: At the end of an injection, the door cannot be closed with a used Enbrel Mini® single-dose prefilled cartridge inside. Reason #2: When AutoTouch experiences a failure, the door will open and remain open. If this occurs, call 1-888-4ENBREL (1-888-436-2735). |
| A chime is repeating but no error lights are showing.
If the door is left open for more than 45 seconds, a chime will sound. Close the door to silence the chime. |
| AutoTouch is not producing chiming sounds.
The sound setting may be off. Turn on by sliding the sound switch down. Enbrel Mini® single-dose prefilled cartridge will not eject. Reason #1: If the viewing window has no light, press the status button to wake up AutoTouch. Then press and hold the door button for at least two seconds to eject. Reason #2: If Enbrel Mini does not eject automatically at the end of an injection, there may be a problem. Call 1-888-4ENBREL (1-888-436-2735). |
| The purple cap is very hard to remove.
The purple cap should not be removed outside of the AutoTouch® reusable autoinjector. It should be removed after it is loaded into AutoTouch, when you are ready to inject. If Enbrel Mini is loaded and the purple cap is difficult to remove, call 1-888-4ENBREL (1-888-436-2735). |
| The injection speed will not change during injection.
Once the injection starts, the speed cannot be changed. Always set the speed prior to injection. |
| The injection is faster or slower than expected.
The speed switch may have been moved unintentionally. Check the speed setting prior to starting each injection. |
Storage & Handling
Storage
AutoTouch® reusable autoinjector
| Do | Do Not |
| Do store AutoTouch in dry, safe place at room temperature such as a cabinet or drawer. | Do not store AutoTouch in the refrigerator with the Enbrel Mini® single-dose prefilled cartridges. |
| Do store AutoTouch in its carton when not in use. | Do not store AutoTouch in extreme heat or cold, or in highly humid environments like the bathroom. |
Handling
AutoTouch reusable autoinjector
| Do | Do Not |
| Do inspect AutoTouch for physical damage or defects before each use. | Do not use AutoTouch if it has been dropped on a hard surface. Do not use AutoTouch if any part appears cracked or broken. |
| Do not leave AutoTouch door open for more than 45 seconds when not in use. (Chime will sound and AutoTouch will go to sleep). | |
| Do not crush, burn, heat, or incinerate the battery as this may cause a risk of fire or explosion. | |
| Do not use AutoTouch if it has been dropped on a hard surface. Call 1-888-4ENBREL (1-888-436-2735) for a replacement. |
Storage and Handling
Enbrel Mini® single-dose prefilled cartridge
| Do | Do Not |
| Do store unused Enbrel Mini in the refrigerator. | Do not freeze the unused Enbrel Mini. Do not warm Enbrel Mini using a heat source such as hot water or a microwave. |
| Do make sure to hold Enbrel Mini with the labeled side facing out and slide into the door. | Do not force Enbrel Mini into the door. Do not use Enbrel Mini if it has been dropped on a hard surface. |
| Do put Enbrel Mini in the door before removing the purple cap. | Do not remove the purple cap before inserting into AutoTouch. Do not re-use or recap Enbrel Mini. |
| Do discard the purple cap immediately after removing to avoid a choking hazard. | Do not use Enbrel Mini if any part appears cracked or broken. |
Cleaning Instructions
Cleaning
AutoTouch® reusable autoinjector
| Do | Do Not |
| Do use an alcohol wipe to clean the injection end of AutoTouch before and after injections. | Do not clean AutoTouch with water. |
| Do use an alcohol wipe to clean all other areas of AutoTouch as desired. | Do not immerse AutoTouch in water. |
| Do not wipe AutoTouch with household cleanser or soap. |
| Warnings |
No modification of the AutoTouch® reusable autoinjector is allowed.
No part of AutoTouch can be repaired or replaced, including the battery.
Do not put anything inside the door other than an Enbrel Mini® single-dose prefilled cartridge.
Do not immerse AutoTouch in water.
Do not reach inside AutoTouch.
Do not crush, burn or heat AutoTouch.
AutoTouch contains moving parts. Keep your fingers out of openings in the injection end or open door.
Keep AutoTouch and Enbrel Mini out of the reach of children.
If AutoTouch fails, the maximum amount of medicine you could receive is the contents of the full Enbrel Mini, which is the correct dose.
Use caution if an error occurs as the needle may be exposed.
Carefully dispose of Enbrel Mini in an FDA approved sharps container.
Call your healthcare provider if you have any concerns regarding an incomplete injection.
When travelling, keep AutoTouch with you, in your carry-on bags.
Do not dispose of AutoTouch in the household trash. Call 1-888-4ENBREL (1-888-436-2735) for a replacement.
| Technical Information |
International Protection Rating
The international protection code for the AutoTouch® reusable autoinjector when stored in its carton is IP52. Which means it is protected from limited dust ingress and from limited dripping water. AutoTouch is not rated for dust or fluid ingress when not stored in its carton.
Environmental Operating Range
AutoTouch will operate in the temperature range of 50° F to 104° F (10° C to 40° C), and 20% to 90% relative humidity, and at elevations from 197 feet below sea level to 11,483 feet above sea level (-60 m to 3500 m).
Environmental Storage Range
Transport and store AutoTouch in its carton, in a dry place at room temperature: 50° F to 104° F (10° C to 40° C). AutoTouch been tested to a brief exposure at -40° F to 158° F (-40° C to 70° C), 50% relative humidity, and pressure equivalent of 14000 ft (4267 m).
Electromagnetic Compatibility
Avoid operating AutoTouch near microwave ovens, wireless routers, baby monitors or other common household electronics that operate using RF transmission. Two meters (six feet) is a reasonable distance.
Avoid operating AutoTouch near high magnetic or other fields such as those around MRI, CAT, or PET scanners.
AutoTouch does not emit RF.
Electrical characteristics
The AutoTouch® reusable autoinjector uses a non-replaceable, non-rechargeable DL123 Lithium battery. The battery has a nominal voltage of 3.0 V with a capacity of 1400 mAh. The expected service life of AutoTouch is two years or 130 injections from the first injection.
AutoTouch has a Type BF applied part: it has a capacitive electrical sensor that is isolated by plastic from the skin.
Dimensions and weight
AutoTouch weighs 0.4 pounds (180 grams), and is 9 inches (228 mm) tall by 1.5 inches (38 mm) wide by 1.8 inches (45 mm) deep.
Biocompatibility and electrical isolation
The injection end of the reusable autoinjector is intended to come into contact with the skin (see Guide to parts). It is a Type BF applied part. This means that it is electrically isolated from the battery. The injection end and finger grip of AutoTouch are made of ABS plastic. This material has been biocompatibility-tested for skin sensitivity and irritation.
| Symbol Table | |
 | Do not re-use |
 | Use-by date (Exp. date) |
 | Lot number |
 | Keep dry |
 | Serial number |
 | Type BF applied part Injection end of device is a Type BF applied part. |
 | CAUTION, consult accompanying documents |
 | Refer to instructions for use |
 | Do not use if package is damaged |
 | This product contains dry natural rubber |
AMGEN®
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
©1998 – 2018 Immunex Corporation. All rights reserved.
[partnumber]
Revised: 12/2018 v5
AutoTouch Connect® reusable autoinjector
for use with
Enbrel Mini® (etanercept)
single-dose prefilled cartridge
User Manual
| Table of Contents | |
| Getting Started | 4 |
| Reference Guide | 5 |
| Resources | 6 |
| Guide to Parts | 8 |
| Troubleshooting: Error Symbols | 18 |
| Troubleshooting: Common Problems | 24 |
| Storage & Handling | 28 |
| Cleaning Instructions | 30 |
| Warnings | 31 |
| What is the Bluetooth® wireless feature? | 32 |
| Technical Information | 36 |
| Symbol Table | 39 |
Getting Started
This User Manual contains helpful information about your AutoTouch Connect® reusable autoinjector (AutoTouch Connect). It includes resources, care details, and a troubleshooting guide to save for ongoing reference.
To learn how to inject using AutoTouch Connect, see your healthcare provider for injection training and use the Instructions for Use included in the carton.
Instructions for Use
A fold-out, step-by-step Instructions for Use is provided in the carton, which provides you with full instructions to learn how to give yourself a safe, successful and accurate injection.
Note: Please read the full Instructions for Use included in the carton.
The Reference Guide on the next page should only be used when you have successfully completed an injection using the Instructions for Use.
Reference Guide
| Prepare and clean your injection site. | Hold the Enbrel Mini® single-dose prefilled cartridge with labeled side facing out and slide into door. Close door. Remove purple cap. | ||||
| 1 | 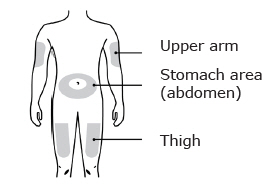 | 2 | 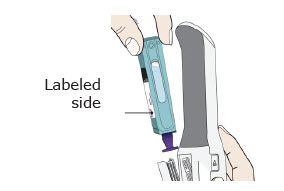 | 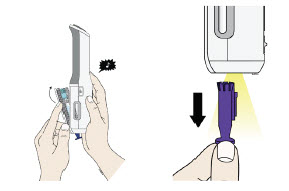 |
|
| Place and hold on skin. Wait for the status button to turn green. | To start injection: Press and release the green status button. | Injection is finished when you hear a chime and all lights are turned off. | |||
| 3 |  | 4 |  | 5 |  |
Resources
ENBREL® SupportPlus provides personalized support services to ENBREL® patients, at no additional cost, including:
- Information about financial support options
- Supplemental injection training with an ENBREL Nurse Partner™
- Educational materials to help support you along the way
Call 1-888-4ENBREL (1-888-436-2735) to enroll or visit {HYPERLINK "http://www.EnbrelSupportPlus.com"} to find out more.
Guide to Parts
AutoTouch Connect® reusable autoinjector
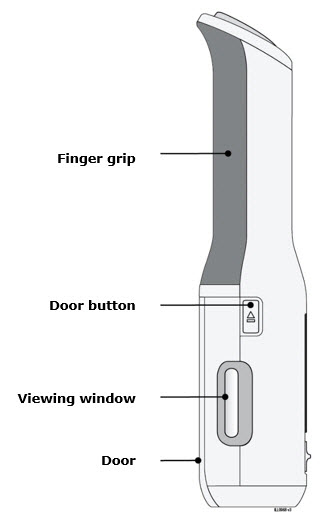
Finger grip
| When injecting, hold the AutoTouch Connect® reusable autoinjector with fingers wrapped around the gray finger grip. |  |
Door button
Press this button to open the door for insertion of the Enbrel Mini® single-dose prefilled cartridge. When your injection is finished, the door will open automatically.
Viewing window
During an injection, you can look through this window and see the plunger lowering to deliver your medicine. When AutoTouch Connect is awake, the viewing window will light up. If Enbrel Mini is in AutoTouch Connect, and the viewing window has no light, then press the status button to wake up AutoTouch Connect.
You will open this door and insert Enbrel Mini into AutoTouch Connect. The door will open automatically when your injection is finished. When inserted properly, Enbrel Mini will slide freely and completely into and out of the door.
| Guide to Parts |
| AutoTouch Connect® reusable autoinjector |
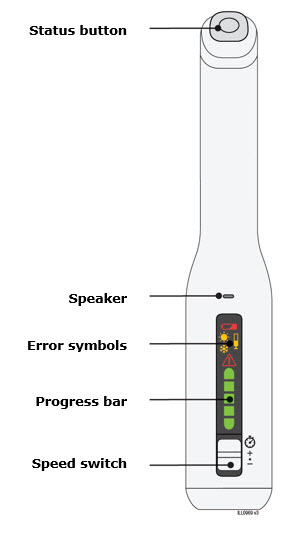 |
Status button
Press this button to begin an injection. Lights will indicate the status of your injection.
Green:
Ready to press and start injection.
Blinking green:
The needle is inserting and your injection is in progress.
Red:
An error has occurred. See the Troubleshooting section of this manual.
Progress bar
These stacked green bars are fully lit when your injection starts, and the bar of lights decrease as your injection is in progress. The lights will disappear when your injection is finished.
Speed switch
The AutoTouch Connect® reusable autoinjector allows you to choose from three injection speeds. The pre-set speed is medium.
(+) is faster
(•) is medium
(–) is slower
| Guide to Parts |
| AutoTouch Connect® reusable autoinjector |
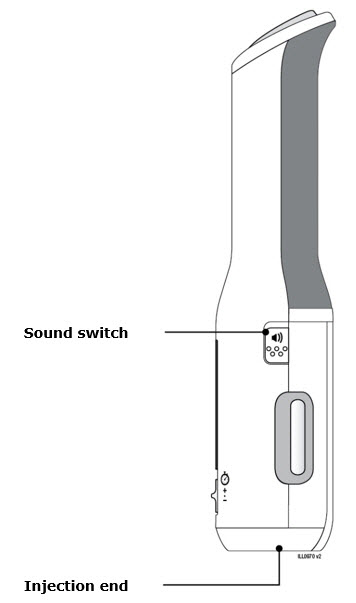 |
Sound switch
Sounds can be turned off and on. (Sounds on) Slide switch down. (Sounds off) Slide switch up so that the red bar is visible.
Note: Error sounds will still be heard if the sounds have been turned off.
Injection end
The entire injection end must be touching your skin during injections. This is also where the needle will come out.
Skin sensor
A skin sensor is located on the injection end. When the injection end is placed on skin the start button light will turn green. Hold the injection end flat and steady on your skin throughout the entire injection process.
Site light
When the Enbrel Mini® single-dose prefilled cartridge is loaded and the purple cap is removed, the injection end will light up to help you see your injection site.
| Guide to Parts |
| Enbrel Mini® single-dose prefilled cartridge |
| Before use |
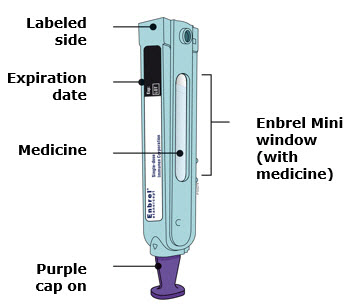 |
| After use |
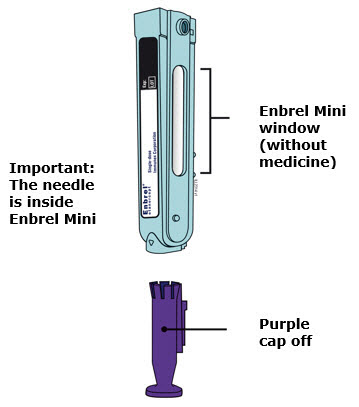 |
Labeled side
When inserting the Enbrel Mini® cartridge make certain that the labeled side is facing out. Then slide Enbrel Mini into the door. It will slide all the way down into the AutoTouch Connect® reusable autoinjector.
Expiration date
Confirm expiration date printed on the label has not passed.
Enbrel Mini window
This is where you will look to examine your medicine before starting an injection. See Instructions for Use for more information.
Purple cap
The purple cap keeps your medicine safe. Do not remove the cap on Enbrel Mini before loading it into AutoTouch Connect. Load Enbrel Mini with the purple cap on, and remove the cap only when you are ready to give yourself the injection. Do not leave the purple cap off for more than five minutes. This can dry out the medicine.
See the Instructions for Use for more detail about loading Enbrel Mini and when to remove the purple cap.
| Guide to Parts |
| AutoTouch Connect® reusable autoinjector |
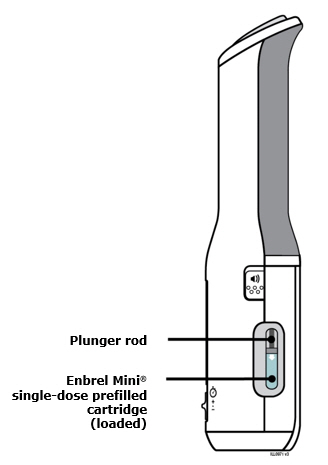 |
How it works.
When you push the status button to start an injection, the AutoTouch Connect® reusable autoinjector pushes a hidden needle out of the Enbrel Mini® single-dose prefilled cartridge into your skin. Then, a plunger rod will push into Enbrel Mini, injecting medicine into your body. After the medicine is injected, the rod will pull back up and the needle will withdraw from your skin. After a successful injection all lights will turn off and the door will open. The needle stays hidden at all times during the injection process.
What you will hear.
Needle insertion and plunger rod are operated by motors. You will hear a motor noise as the rod moves down and then back up again. Whenever you hear a motorized noise, you will know that an injection is in progress.
What you will see.
During the injection, the viewing window light will be on, and the plunger rod will move through the window. Also during the injection, the status light will be flashing green, and the progress bar will decrease. After a successful injection, the progress bar, and status button will turn off, and AutoTouch Connect will open the door.
If the status light turns red, and beeps for more than a few seconds, an error has occurred. Use the troubleshooting section of this manual to see what to do if this happens.
Troubleshooting:
Error Symbols
Error Symbols
The AutoTouch Connect® reusable autoinjector makes a chime sound, lights the status button red, and displays an error symbol if there is a problem.
See the following description of each error symbol, possible reasons for the error, and actions you can take.
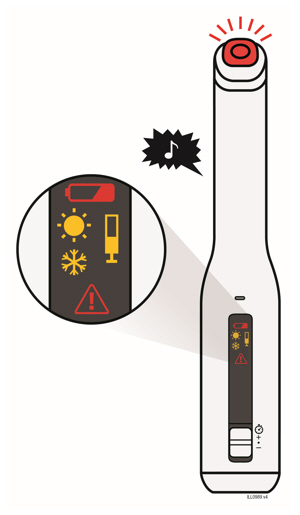
 Low battery
Low battery
Symbol: Red battery and error sound.
Problem: The battery is running very low. Less than three weeks or three injections remain. The battery is not replaceable or rechargeable.
Action: Call 1-888-4ENBREL (1-888-436-2735) for a replacement AutoTouch Connect® reusable autoinjector.
 Battery dead
Battery dead
Symbol: Red battery and red triangle with exclamation point, red status button, and error sound.
Problem: The AutoTouch Connect reusable autoinjector battery is dead.
Action: Call the 1-888-4ENBREL (1-888-436-2735) for a replacement AutoTouch Connect reusable autoinjector.
 Too hot or cold
Too hot or cold
Symbol: Orange sun and a snowflake, and an error sound.
Problem: AutoTouch Connect is too hot or too cold. This will lock the door.
Action: Place AutoTouch Connect at room temperature in a safe, dry place and allow it to naturally cool or warm to room temperature. Then try again. Store the AutoTouch Connect reusable autoinjector in a dry, safe place, such as a cabinet or drawer at 50 °F to 104 °F (10 °C to 40 °C).
Troubleshooting:
Error Symbols
 Enbrel Mini® problem
Enbrel Mini® problem
Symbol: Orange Enbrel Mini single-dose prefilled cartridge with blinking red status button and error alert sound.
Problem: There are five possible reasons you are getting this error. Please read below.
Problem #1: The purple cap was removed before inserting Enbrel Mini.
Action: Wait for the error to clear and close the door without Enbrel Mini in, and try again with a new Enbrel Mini. Make sure to leave the purple cap on until Enbrel Mini has been placed inside the AutoTouch Connect® reusable autoinjector and the door is closed. Call 1-888-4ENBREL (1-888-436-2735) for a replacement Enbrel Mini.
Problem #2: Sensor lost skin contact during injection.
Action: Hold the injection end of AutoTouch Connect on your skin throughout the entire injection until the green status button turns off, even if the motor sounds stop. Lifting or shifting on your skin during injection may lead to an incomplete injection. Call your healthcare provider if you feel you have given yourself an incomplete injection.
Problem #3: Enbrel Mini® single-dose prefilled cartridge is defective or not recognized as an Amgen product.
Action: Remove Enbrel Mini. Replace with a new Enbrel Mini. Call 1-888-4ENBREL (1-888-436-2735) for a replacement Enbrel Mini.
Problem #4: Door is held closed for more than one minute.
Action: Remove your hand from the door. When the door opens, remove Enbrel Mini from AutoTouch Connect®, if present. Wait for the error symbol to stop blinking and close the door. If you have not given yourself an injection, place a new Enbrel Mini in AutoTouch Connect and continue. If the error symbol remains on, call 1-888-4ENBREL (1-888-436-2735).
Problem #5: AutoTouch Connect has been dropped.
Action: A dropped AutoTouch Connect is not safe to use. Call 1-888-4ENBREL (1-888-436-2735) for a replacement.
Troubleshooting:
Error Symbols
 Needle Exposure
Needle Exposure
Symbol: Orange Enbrel Mini® single-dose prefilled cartridge, a red triangle with an exclamation point, red status button, and error sound.
Problem: A problem occurred during an injection and the needle may be exposed.
Action: If there is still fluid in Enbrel Mini, an incomplete dose may have been injected. Call your healthcare provider if you feel you have given yourself an incomplete injection. Call 1-888-4ENBREL (1-888-436-2735) for further assistance with your AutoTouch Connect®.
Use caution when both Enbrel Mini problem symbol and the AutoTouch Connect reusable autoinjector failure symbols are lit as the needle may be exposed. Take special care when removing and handling Enbrel Mini. Remove Enbrel Mini then put it in an FDA-cleared sharps disposal container.
 AutoTouch Connect® Reusable Autoinjector Failure
AutoTouch Connect® Reusable Autoinjector Failure
Symbol: Red triangle with an exclamation point, red status button, and error sound.
Problem: Several errors have occurred or AutoTouch Connect has stopped working.
Action: Reset AutoTouch Connect.
To reset AutoTouch Connect:
Hold AutoTouch Connect away from skin and press the status button to wake AutoTouch Connect. The failure symbol should begin blinking and a chime should sound.
While the failure symbol is blinking, press and hold door button until all symbols are temporarily displayed and the status button blinks green. The door button should be held for at least 10 seconds. After a successful reset, if an Enbrel Mini® single-dose prefilled cartridge is still inside AutoTouch Connect, remove it. Close the AutoTouch Connect door. Then, for the next injection, start by pressing the door button to open Enbrel Mini door.
If AutoTouch Connect does not respond after three attempts to reset, call 1-888-4ENBREL (1-888-436-2735).
Troubleshooting:
Common Problems
Common Problems
Enbrel Mini® single-dose prefilled cartridge is difficult to insert into the door.
Never force Enbrel Mini into the door. When positioned correctly, it will fall freely and completely into and out of the door. If it is difficult to load, double check that you are holding Enbrel Mini as shown.
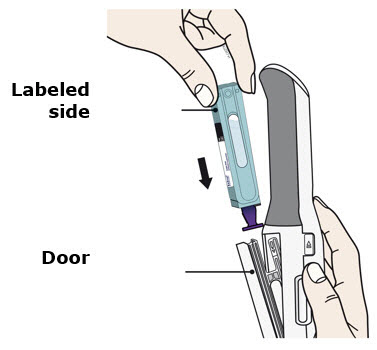
- Purple cap is facing down
- Label facing outwards (away from the handle)
Injection does not start when pressing the status button.
Reason #1: Place the AutoTouch Connect® reusable autoinjector on your skin and wait for the status button to turn green. A skin sensor is located on the injection end. You cannot start an injection unless the injection end of AutoTouch Connect is touching your skin. Hold the injection end on your skin throughout the entire injection.
Reason #2: AutoTouch Connect may be asleep. To conserve battery power, AutoTouch Connect goes into a "sleep mode" after three minutes of no activity. If it seems unresponsive/asleep, remove AutoTouch Connect from your skin and press the status button to wake it up.
An Enbrel Mini® error symbol appears immediately after loading the Enbrel Mini single-dose prefilled cartridge.
Reason: This will happen if the purple cap has been removed before inserting Enbrel Mini into the door. Do not reuse or recap. Begin again using a new Enbrel Mini.
Do not remove the purple cap until after Enbrel Mini has been inserted into AutoTouch Connect.
Troubleshooting:
Common Problems
Injection aborts or an error symbol appears while the injection is in process.
The AutoTouch Connect® reusable autoinjector will abort an injection if the sensor loses skin contact. Avoid adjusting or moving AutoTouch Connect during an injection. Hold AutoTouch Connect on your skin until the green status light turns off and the injection is finished. Shifting, repositioning or lifting from your skin during injection may lead to an incomplete dose.
AutoTouch Connect door will not remain closed.
Reason #1: At the end of an injection, the door cannot be closed with a used Enbrel Mini® single-dose prefilled cartridge inside.
Reason #2: When AutoTouch Connect experiences a failure, the door will open and remain open. If this occurs, call 1-888-4ENBREL (1-888-436-2735).
A chime is repeating but no error lights are showing.
If the door is left open for more than 45 seconds, a chime will sound. Close the door to silence the chime.
AutoTouch Connect is not producing chiming sounds.
The sound setting may be off. Turn on by sliding the sound switch down.
Enbrel Mini® single-dose prefilled cartridge will not eject.
Reason #1: If the viewing window has no light, press the status button to wake up AutoTouch Connect®. Then press and hold the door button for at least two seconds to eject.
Reason #2: If Enbrel Mini does not eject automatically at the end of an injection, there may be a problem. Call 1-888-4ENBREL (1-888-436-2735).
The purple cap is very hard to remove.
The purple cap should not be removed outside of the AutoTouch Connect reusable autoinjector. It should be removed after it is loaded into AutoTouch Connect, when you are ready to inject. If Enbrel Mini is loaded and the purple cap is difficult to remove, call 1-888-4ENBREL (1-888-436-2735).
The injection speed will not change during injection.
Once the injection starts, the speed cannot be changed. Always set the speed prior to injection.
The injection is faster or slower than expected.
The speed switch may have been moved unintentionally. Check the speed setting prior to starting each injection.
Storage & Handling
Storage
AutoTouch Connect® reusable autoinjector
| Do | Do Not |
|---|---|
| Do store AutoTouch Connect in dry, safe place at room temperature such as a cabinet or drawer. | Do not store AutoTouch Connect in the refrigerator with the Enbrel Mini® single-dose prefilled cartridges. |
| Do store AutoTouch Connect in its carton when not in use. | Do not store AutoTouch Connect in extreme heat or cold, or in highly humid environments like the bathroom. |
Handling
AutoTouch Connect reusable autoinjector
| Do | Do Not |
|---|---|
| Do inspect AutoTouch Connect for physical damage or defects before each use. | Do not use AutoTouch Connect if it has been dropped on a hard surface. Do not use AutoTouch Connect if any part appears cracked or broken. |
| Do not leave AutoTouch Connect door open for more than 45 seconds when not in use. (Chime will sound and AutoTouch Connect will go to sleep). | |
| Do not crush, burn, heat, or incinerate the battery as this may cause a risk of fire or explosion. | |
| Do not use AutoTouch Connect if it has been dropped on a hard surface. Call 1-888-4ENBREL (1-888-436-2735) for a replacement. |
Storage and Handling
Enbrel Mini® single-dose prefilled cartridge
| Do | Do Not |
|---|---|
| Do store unused Enbrel Mini in the refrigerator. | Do not freeze the unused Enbrel Mini. Do not warm Enbrel Mini using a heat source such as hot water or a microwave. |
| Do make sure to hold Enbrel Mini with the labeled side facing out and slide into the door. | Do not force Enbrel Mini into the door. Do not use Enbrel Mini if it has been dropped on a hard surface. |
| Do put Enbrel Mini in the door before removing the purple cap. | Do not remove the purple cap before inserting into AutoTouch Connect®. Do not re-use or recap Enbrel Mini. |
| Do discard the purple cap immediately after removing to avoid a choking hazard. | Do not use Enbrel Mini if any part appears cracked or broken. |
Cleaning Instructions
Cleaning
AutoTouch Connect® reusable autoinjector
| Do | Do Not |
|---|---|
| Do use an alcohol wipe to clean the injection end of AutoTouch Connect before and after injections. | Do not clean AutoTouch Connect with water. |
| Do use an alcohol wipe to clean all other areas of AutoTouch Connect as desired. | Do not immerse AutoTouch Connect in water. |
| Do not wipe AutoTouch Connect with household cleanser or soap. |
Warnings
No modification of the AutoTouch Connect® reusable autoinjector is allowed.
No part of AutoTouch Connect can be repaired or replaced, including the battery.
Do not put anything inside the door other than an Enbrel Mini® single-dose prefilled cartridge.
Do not immerse AutoTouch Connect in water.
Do not reach inside AutoTouch Connect.
Do not crush, burn or heat AutoTouch Connect.
AutoTouch Connect contains moving parts. Keep your fingers out of openings in the injection end or open door.
Keep AutoTouch Connect and Enbrel Mini out of the reach of children.
If AutoTouch Connect fails, the maximum amount of medicine you could receive is the contents of the full Enbrel Mini, which is the correct dose.
Use caution if an error occurs as the needle may be exposed.
Carefully dispose of Enbrel Mini in an FDA approved sharps container.
Call your healthcare provider if you have any concerns regarding an incomplete injection.
When travelling, keep AutoTouch Connect with you, in your carry-on bags.
Do not dispose of AutoTouch Connect in the household trash. Call 1-888-4ENBREL (1-888-436-2735) for a replacement.
What is the Bluetooth® wireless feature?
This AutoTouch Connect® reusable autoinjector has an optional Bluetooth® wireless feature to automatically record your injections. This wireless feature will not affect your injection steps. Whether or not you use this wireless feature, please make sure you follow steps in the Instructions for Use. To use this feature, download and set up the Embark® − Patient Support app. After Embark is set up and paired with AutoTouch Connect, follow all injection instructions as you normally would.
AutoTouch Connect transmits a signal to the Embark app on your mobile device and keeps track of your Enbrel injections.
For questions about the Embark app and this feature, go to www.enbrel.com.
Remember, this feature is optional and you can inject without using the Embark app. Always follow all steps found in the Instructions for Use.
Embark – Patient Support app
 | The Embark app is a medication reminder that allows you to schedule and record your injections using your mobile device. |
With Embark you can:
- Set up medication and appointment reminders.
- Record your Enbrel injection sites.
- Sign up for ENBREL® SupportPlus programs.
- Access Enbrel educational materials.
Pairing AutoTouch Connect®
When setting up the Embark® app, bring AutoTouch Connect next to your mobile device, then open and close the AutoTouch Connect door.
The Embark app will guide you through pairing your AutoTouch Connect reusable autoinjector. The AutoTouch Connect uses standard Bluetooth® authentication and encryption for its wireless data security.
Once paired, AutoTouch Connect will try to sync with the Embark app for up to 48 hours after every injection. To sync at any time, bring AutoTouch Connect next to your mobile device, then open and close the AutoTouch Connect door.
No patient information is stored.
What to do if you lose your phone or get a new phone
The Embark app can only be synced to one mobile device at a time. If you lose your mobile device (or get a new one), you can download and log in to the Embark app on your new mobile device.
You can sync AutoTouch Connect to your new mobile device and retrieve all your previous injection history, reminders, and notifications on the Embark app.
Once you have synced the Embark app to your new mobile device, you cannot access the Embark app on your old mobile device. You will not receive medication, refill, and appointment reminders from the Embark app on your old mobile device.
Important Information Required by the Federal Communications Commission (FCC)
FCCID: 2AGZ4–ATCONNECT
The AutoTouch Connect® reusable autoinjector contains a Bluetooth® wireless feature.
AutoTouch Connect complies with Part 15 of the FCC Rules.
Operation is subject to the following two conditions:
- AutoTouch Connect may not cause harmful interference.
- AutoTouch Connect must accept any interference received, including interference that may cause undesirable operation.
Modification to AutoTouch Connect shall not be made without the written consent of Amgen.
Unauthorized modification may void the authority granted under FCC rules permitting the operation of AutoTouch Connect.
AutoTouch Connect has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. AutoTouch Connect generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation.
If AutoTouch Connect® does cause harmful interference to radio or television reception, which can be determined by moving AutoTouch Connect far away and back, the user is encouraged to try to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between AutoTouch Connect and interference receiver.
- Consult the dealer or an experienced radio/TV technician for help.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth Special Interest Group (SIG) Inc. and any use of such marks by Amgen is under license. Other trademarks and trade names are those of their respective owners.
Technical Information
International Protection Rating
The international protection code for the AutoTouch Connect® reusable autoinjector when stored in its carton is IP52. Which means it is protected from limited dust ingress and from limited dripping water. AutoTouch Connect is not rated for dust or fluid ingress when not stored in its carton.
Environmental Operating Range
AutoTouch Connect will operate in the temperature range of 50 °F to 104 °F (10 °C to 40 °C), and 20% to 90% relative humidity, and at elevations from 197 feet below sea level to 11,483 feet above sea level (-60 m to 3500 m).
Environmental Storage Range
Transport and store AutoTouch Connect in its carton, in a dry place at room temperature: 50 °F to 104 °F (10 °C to 40 °C). AutoTouch Connect has been tested to a brief exposure at -40 °F to 158 °F (-40 °C to 70 °C), 50% relative humidity, and pressure equivalent of 14,000 feet (4267 m).
Bluetooth® Characteristics
The AutoTouch Connect® is designed to communicate with the Embark® app running on a Bluetooth enabled mobile device. The Bluetooth technology in the AutoTouch Connect complies with the Bluetooth Core Specification v5.0 and is qualified by Bluetooth SIG. The AutoTouch Connect has been designed to transmit default 0dBm standard industrial, scientific and medical (ISM) 2.4 GHz band radio frequency (RF) and covers up to 10 meters communication range.
Quality of Service and Security Information
The AutoTouch Connect autoinjector establishes an authenticated and encrypted connection with the Embark app running on a mobile device. Initial setup requires physical access to the autoinjector labeled serial number for user input at initial pairing/secure bonding. The app will walk you through this setup process.
Data is transferred from the autoinjector on a one-to-one paired mobile device for ensuring data integrity and quality of service (QoS). Data transferred is read-only and no patient information is stored on the autoinjector.
Data can be sent at any time, and temporary loss of proper QoS will be resolved upon resumed connection to the app. If connection with the app drops and re-establishes, data transfer resumes with data integrity verification. Data transfer latency or asynchronization does not affect drug delivery tracking as data will be confirmed upon reconnection.
Electromagnetic Compatibility
Portable and mobile RF communications equipment can affect medical electrical equipment. Avoid operating AutoTouch Connect® near microwave ovens, wireless routers, baby monitors or other common household electronics that operate using RF transmission, including RFID emitters. A minimum distance of 30 cm (12 inches) is recommended.
Avoid operating AutoTouch Connect near high magnetic or other fields such as those around MRI, CAT, or PET scanners.
AutoTouch Connect emits RF.
Electromagnetic Emissions
| The autoinjector is intended for use in the electromagnetic environment specified below. The user of the autoinjector should ensure that it is used in such an environment. | ||
|---|---|---|
| Emissions Test | Compliance | Electromagnetic Environment - Guidance |
| RF Emissions (CISPR 11) | Group 1 | The autoinjector uses RF energy for its internal and system interface functions. Its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. |
| RF Emissions (CISPR 11) | Class B | The autoinjector is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. |
| Harmonic emissions IEC 61000-3-2 | Not Applicable | |
| Voltage fluctuations/flicker emissions IEC 61000-3-3 | Not Applicable | |
Electromagnetic Immunity
| The autoinjector is intended for use in the electromagnetic environment specified below. The user of this autoinjector should ensure that it is used in such an environment. | |||
|---|---|---|---|
| Immunity Test | IEC 60601 Test Level | Compliance Level | Electromagnetic Environment-Guidance |
| Electrostatic Discharge (ESD) IEC 61000-4-2 | ±8 kV Contact ±15 kV Air | ±15 kV Contact ±20 kV Air | Floors should be wood, concrete or ceramic tile. If floors are synthetic, the relative humidity should be at least 30%. |
| Power Frequency 50/60 Hz Magnetic Fields IEC 61000-4-8 | 30 A/m | 30 A/m at 60 Hz | Power frequency magnetic fields should be that of typical commercial or hospital environment. |
| RF Electro-magnetic Field IEC 61000-4-3 | 10 V/m 80 MHz – 2.5 GHz | (E1) = 10 V/m 26 MHz – 2.7 GHz | Portable and mobile RF communications equipment should be separated from the device by no less than the distances of 30 cm. |
Electrical characteristics
The AutoTouch Connect® reusable autoinjector uses a non-replaceable, non-rechargeable DL123 Lithium battery. The battery has a nominal voltage of 3.0 V with a capacity of 1400 mAh.
The autoinjector enclosure is a Type BF applied part: The skin sensor and other electronics are isolated from the skin.
Dimensions and weight
AutoTouch Connect weighs 0.4 pounds (180 grams), and is 9 inches (228 mm) tall by 1.5 inches (38 mm) wide by 1.8 inches (45 mm) deep.
Biocompatibility and electrical isolation
The autoinjector enclosure is intended to come into contact with the skin (see Guide to Parts). It is a Type BF applied part. This means that it is electrically isolated from the battery. The injection end and finger grip of AutoTouch Connect® are made of ABS plastic. This material has been biocompatibility-tested for skin sensitivity and irritation.
Contraindications
This device is contraindicated for use in a Magnetic Resonance (MR) environment. For contraindications of ENBREL® please refer to ENBREL prescribing information.
| Symbol Table | |
|
| Do not re-use |
|
| Use-by date (Exp. date) |
|
| Lot number |
|
| Keep dry |
|
| Serial number |
|
| Type BF applied part The autoinjector enclosure is a Type BF applied part. |
|
| CAUTION, consult accompanying documents |
|
| Refer to instructions for use |
|
| Do not use if package is damaged |
|
| Magnetic Resonance (MR) Unsafe |
AMGEN®
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
©2021-2023 Immunex Corporation.
All rights reserved.
[partnumber]
Revised: 06/2023 v2
 This printed material is recyclable.
This printed material is recyclable.
Instructions for Use
Enbrel® (en-brel)
(etanercept)
injection, for subcutaneous use
single-dose vial
Read this Instructions for Use before you start taking Enbrel and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition and treatment.
Step 1: Set up

1a. Strength and dose
The strength of the Enbrel single-dose vial is different than the Enbrel multiple-dose vial.
Each single-dose vial contains 25 mg (0.5 mL).
Review your prescription carefully for dose and dosing schedule. Only inject Enbrel after you or your caregiver has received training by your healthcare provider. Your healthcare provider will tell you how often you should use Enbrel. Do not use Enbrel more often than directed by your healthcare provider. If you forget to use Enbrel, inject your dose right away. Inject your next dose at your regular scheduled time. If you do not know when to inject Enbrel, call your healthcare provider or pharmacist.
Keep Enbrel out of the reach of children.

1b. Store Enbrel single-dose vials
Store Enbrel in the refrigerator between 36°F to 46°F (2°C to 8°C). You may also store the Enbrel single-dose vials at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days. Throw away Enbrel that has been stored at room temperature after 30 days.
Do not shake.
Do not freeze or store in extreme heat or cold.
Store Enbrel in the original carton to protect from light or damage.
| If your dose is 0.5 mL, or LESS |
|
| Remove 1 single-dose vial | |
| If your dose is MORE than 0.5 mL |
|
| Remove 2 single-dose vials |
1c. Remove correct number of vials
Remove the correct number of Enbrel single-dose vials from the original carton.
Check your prescription to determine if your dose will require 1 or 2 single-dose vials.
Your dose is determined in milliliters (mL).
Your child's dose of Enbrel depends on his or her weight. Your child's healthcare provider will tell you which form of Enbrel to use and how much to give your child.
| Green vial cap WAIT |
|
|
1d. Inspect the single-dose vial(s)
Leave the single-dose vial(s) at room temperature, with the green cap(s) on, for at least 30 minutes.
Enbrel is clear and colorless. There may be small white particles in the solution. Check the expiration date.
Keep out of direct sunlight.
Do not use Enbrel if:
- the expiration date has passed
- the green cap is not attached
- it has lumps, is discolored, or is cloudy.
If there are any issues with your Enbrel single-dose vials, please call 1-888-4ENBREL (1-888-436-2735).
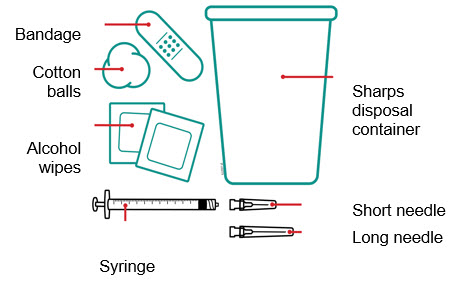
1e. Gather all materials and wash hands
Wash your hands with soap and water.
Place the following items on a clean well-lit, flat surface:
- 1 mL syringe
- Long needle: for withdrawal
- Short needle: for injection
- Alcohol wipes
- Cotton balls
- Bandage
- Sharps disposal container
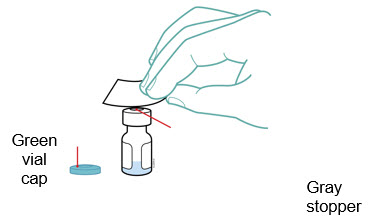
1f. Pop off the green vial cap then wipe the gray stopper
Use an alcohol wipe to clean the gray stopper.
If you require a second single-dose vial, clean the gray stopper of the second single-dose vial with a new alcohol wipe.
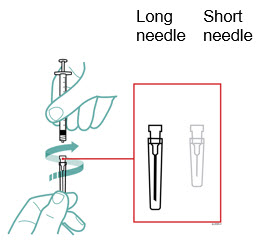
1g. Attach the long needle to the syringe
Twist the long needle onto the syringe.
Step 2: Prepare dose
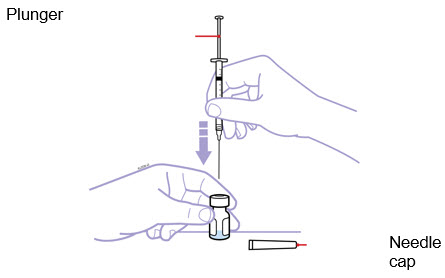
2a. Pull plunger back, insert needle, and push air into single-dose vial
Pull the needle cap straight off and away from your body. Save the needle cap for later.
Pull the syringe plunger back to 0.5 mL.
Hold the single-dose vial on a flat surface with 1 hand. Insert the long needle through the gray rubber stopper above the medicine in the single-dose vial.
Slowly push 0.5 mL of air into the single-dose vial.
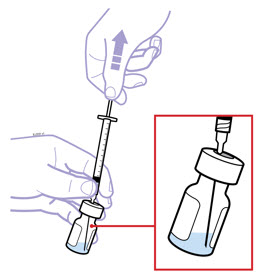
2b. Tilt the single-dose vial to withdraw all medicine
Slowly pull back the plunger to fill the syringe with all the medicine from the single-dose vial.
Remove needle from the single-dose vial.
The air in the syringe will be removed later.
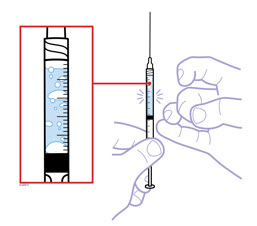
2c. Gently tap the syringe with fingers to release bubbles
Gently tap the syringe with your fingers to release air pockets and bubbles until they rise to the top of the syringe.
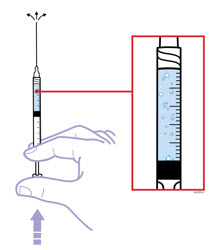
2d. Push out large air pockets and bubbles
After you have gently tapped the large air pockets and bubbles to the top of the syringe, push the plunger up to remove the air out of the syringe.
Small amounts of tiny air bubbles are ok.
If you need 1 single-dose vial, push the plunger to your total prescribed dose and continue to step 2g.
If you need more than 1 single-dose vial to get your total prescribed dose, follow these 2 steps:
|
| 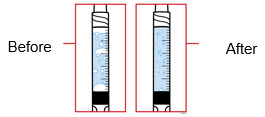 |
| 2e. Insert the same needle in the second vial Tilt vial to withdraw medicine. Slowly pull back the plunger to fill the syringe with all medicine from the vial. Remove the needle from the single-dose vial. The air will be removed in the next step | 2f. Gently tap the syringe to remove the air Hold the needle pointing up and gently tap the syringe so air bubbles rise to the top. Push the plunger to your prescribed dose. Small amounts of tiny air bubbles are ok. |
|
| |
| 2g. Use 1-handed scoop method to recap For your safety, place the needle cap on a flat surface. |
Using 1 hand, slide the needle into the cap and scoop upwards to cap the needle, without using your other hand.
Then use your other hand to secure the cap and snap into place.
Step 3: Inject and throw away
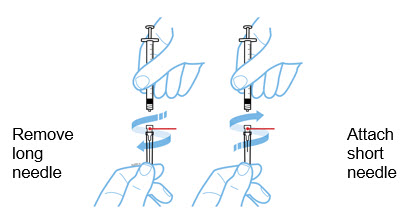
3a. Replace the long needle with the short needle
Twist the long needle off of the syringe.
Throw away the long needle in the sharps disposal container.
Twist the short needle onto the syringe.
Do not remove the needle cover until you are ready to inject.
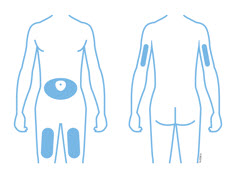
3b. Choose and clean the injection site
- thigh or stomach (avoid 2 inches around the navel)
- back of upper arm (only if someone else is giving the injection)
Choose a different site each time you give yourself an injection. Avoid injecting into tender, raised, red or scaly skin.
Clean the injection site with an alcohol wipe and let dry. Do not touch this area again before injecting.
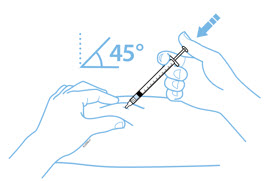
3c. Pinch and inject Enbrel
Gently pinch skin. With a quick firm action, insert the needle into your skin at a 45-degree angle.
When the needle is completely inserted into the skin, slowly push the plunger all the way down.
When the syringe is empty, remove the needle and syringe from your skin. Do not recap the needle.
Do not rub the injection site. If you see drops of blood at the injection site, you can press a cotton ball over the injection site until bleeding has stopped. Apply an adhesive bandage, if needed.
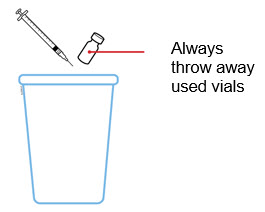
3d. Throw away the used single-dose vials, needles and syringe
Single-dose vials do not contain preservatives and are for 1-time use only. Unused medicine in the single-dose vials must be thrown away in a sharps disposal container.
Additional disposal information
Do not throw away the vials, needles, and syringe in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away your sharps disposal container. There may be state or local laws about how you should throw away used vials, needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not throw away your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Do not reuse the vials, syringe, or needles.
- Do not recycle the vials, syringe, needles, or sharps disposal container or throw them into household trash.
Important: Always keep the sharps disposal container out of the reach of children.
If you experience any difficulty using your Enbrel single-dose vials, please call 1-888-4ENBREL (1-888-436-2735).
If you have any questions about your Enbrel dosing, please call your healthcare provider.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMGEN®
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
U.S. License Number 1132
© 1998-2020, 2022 Immunex Corporation. All rights reserved.
[part number]
Revised: 06/2022 v2
 This printed material is recyclable.
This printed material is recyclable.
Front Panel
| Read these instructions before using Enbrel®
Single-Dose Vial Instructions for Use |
REFERENCE GUIDE
| Side 1 | Reference Guide
Enbrel® (en-brel) (etanercept) injection, for subcutaneous use Single-Dose Prefilled SureClick® Autoinjector 50 mg/mL | Read all instructions in carton before use. Questions? Call 1-888-4ENBREL (1-888-436-2735) | (Material #, Code 128 w/ Human Readable) |
Guide to parts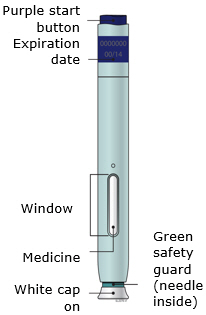 | Wait at least 30 minutes for the autoinjector to come to room temperature before giving your injection. | Pull the white cap straight off only when you are ready to inject. Do not leave the white cap off for more than 5 minutes. This can dry out the medicine. |
|
| Prepare and clean your injection site. |
2 |
||
1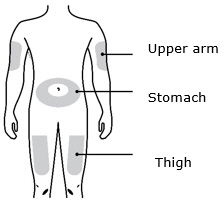 | 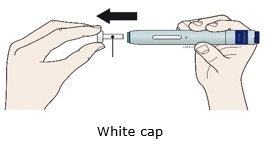 | 5 minutes
 |
|
| Turn over to continue | |||
| Side 2 | Reference Guide
Enbrel® (en-brel) (etanercept) injection, for subcutaneous use Single-Dose Prefilled SureClick® Autoinjector 50 mg/mL | AMGEN® | Read other side first | ||
| Manufactured by: Immunex Corporation Thousand Oaks, CA 91320-1799 © 1998, 2019, 2021-2022 Immunex Corporation. All rights reserved. <part number> Revised: 8/2022 v9
|
|||||
| Stretch or pinch your injection site to create a firm surface. | Put the green safety guard (needle inside) on your skin at 90 degrees. | Firmly push down the autoinjector onto your skin until it stops moving. | Keep pushing down on your skin for about 15 seconds. | ||
| 3 | 4 | 5 | 6 | ||
| Stretch method: |
| 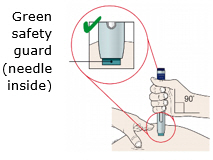 | When you are ready to inject, press the purple start button. You will hear a click. | 15 seconds
 Window turns from clear to yellow when the injection is done. |
|
| Pinch method: | or
| 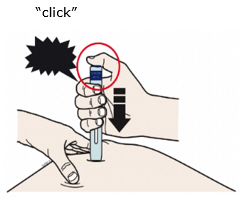 | 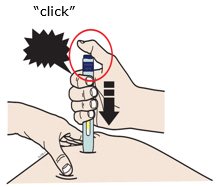 |
||
PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-455-04
Enbrel®
etanercept
25 mg/0.5 mL
Single-Dose Prefilled Syringe
25 mg/0.5 mL
Attention: Not for use in pediatric patients under 31 kg (68 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 single-dose prefilled syringes, 1 package
insert with attached Medication Guide) are intended to be
dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required
for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320

PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-435-04
Enbrel®
etanercept
50 mg/mL
Single-Dose Prefilled Syringe
50 mg/mL
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 single-dose prefilled syringes, 1 package
insert with attached Medication Guide) are intended to be
dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required
for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320

PRINCIPAL DISPLAY PANEL - 25 mg/0.5 mL Syringe Carton
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-010-04
Enbrel®
etanercept
25
mg/0.5 mL
Not made with
natural rubber latex
25 mg/0.5 mL
Single-Dose Prefilled Syringe
Attention: Not for use in pediatric patients under 31 kg (68 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Each single-dose prefilled syringe contains 0.5 mL of a clear and
colorless solution containing 25 mg/0.5 mL etanercept and is formulated at
pH 6.3 ± 0.2, with 25 mM L-arginine hydrochloride, 120 mM sodium chloride,
and 1% sucrose.
Specific activity: approximately 1.7 x 106 U/mg.
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
U.S. License No. 1132
©2013, 2017, 2022-2023 Immunex Corporation
Patent: http://pat.amgen.com/enbrel/
This carton is recyclable.

PRINCIPAL DISPLAY PANEL - 50 mg/mL Syringe Carton
Contains 4 Single-Dose Prefilled Syringes
NDC 58406-021-04
Enbrel®
etanercept
50
mg/mL
Not made with
natural rubber latex
50 mg/mL
Single-Dose Prefilled Syringe
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Each single-dose prefilled syringe contains 1 mL of a clear and
colorless solution containing 50 mg/mL etanercept and is formulated at
pH 6.3 ± 0.2, with 25 mM L-arginine hydrochloride, 120 mM sodium chloride,
and 1% sucrose.
Specific activity: approximately 1.7 x 106 U/mg.
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
U.S. License No. 1132
©2013, 2017, 2022-2023 Immunex Corporation
Patent: http://pat.amgen.com/enbrel/
This carton is recyclable.
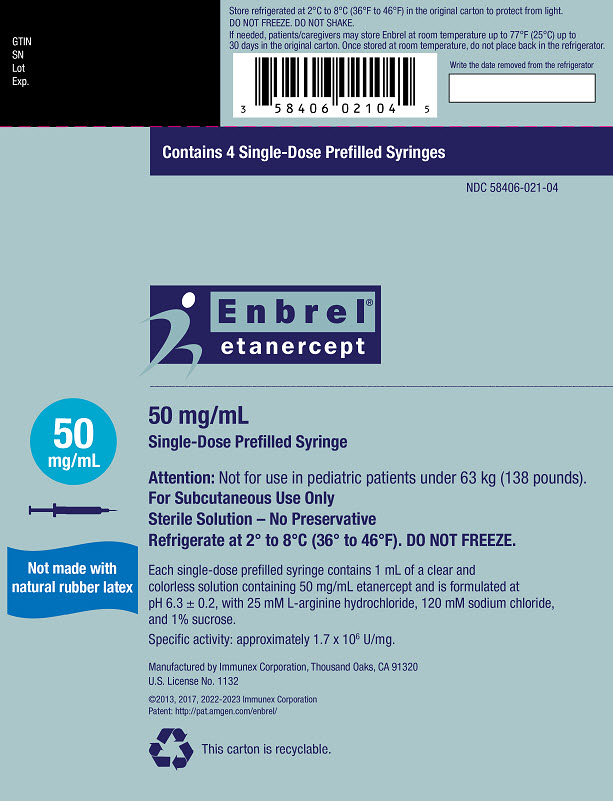
PRINCIPAL DISPLAY PANEL
Contains 4 Single-Dose Prefilled Autoinjectors
NDC 58406-445-04
Enbrel®
etanercept
SureClick® Autoinjector
50 mg/mL
Single-Dose Prefilled Autoinjector
50 mg/mL
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton Contents (4 prefilled SureClick® Autoinjectors, 1 package insert with
attached Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320-1799

PRINCIPAL DISPLAY PANEL - 50 mg/mL Autoinjector Carton
Contains 4 Single-Dose Prefilled Autoinjectors
NDC 58406-032-04
Enbrel®
etanercept
50
mg/mL
Not made with
natural rubber latex
SureClick® Autoinjector
50 mg/mL
Single-Dose Prefilled Autoinjector
Attention: Not for use in pediatric patients under 63 kg (138 pounds).
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Each single-dose prefilled autoinjector contains 1 mL of a clear and colorless solution containing
50 mg/mL etanercept and is formulated at pH 6.3 ± 0.2, with 25 mM L-arginine hydrochloride,
120 mM sodium chloride, and 1% sucrose.
Specific activity: approximately 1.7 x 106 U/mg.
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320-1799
U.S. License No. 1132
©2015, 2017, 2022-2023 Immunex Corporation
Patent: http://pat.amgen.com/enbrel/
This carton is recyclable.
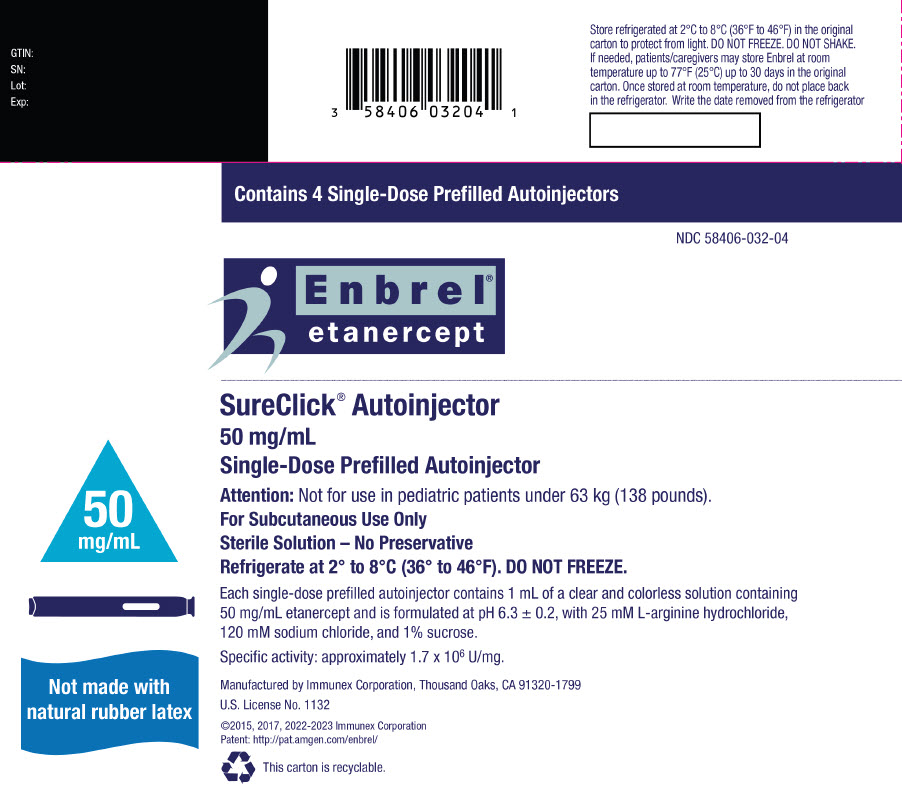
PRINCIPAL DISPLAY PANEL
Contains 4 Multi-Dose Trays
NDC 58406-425-34
AMGEN®
Enbrel®
etanercept
25 mg/vial
Multiple-Dose Vial
See package insert for full prescribing information
and instructions for preparation and administration.
25 mg/vial
Each vial contains a sterile lyophilized preparation
of 25 mg etanercept (a recombinant CHO cell-derived
product), 40 mg mannitol, 10 mg sucrose, and
1.2 mg tromethamine.
Specific activitiy: approximately 1.7 x 106 U/mg.
No U.S. standard of potency. Volume after reconstitution
with 1 mL diluent is 1 mL.
Before and after reconstitution refrigerate
at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
For Subcutaneous Use Only
AMGEN®
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
Contains diluent syringes (Made in Germany)
U.S. License No. 1132
©2013, 2016 Immunex Corporation
Patent: http://pat.amgen.com/enbrel/
PRINCIPAL DISPLAY PANEL
Contains 4 Single-dose prefilled cartridges
NDC 58406-456-04
Enbrel®
etanercept
50 mg/mL
Enbrel Mini™ prefilled cartridge
50 mg/mL
Single-dose prefilled cartridge
For use with AutoTouch™ reusuable autoinjector only
Attention: Not for use in pediatric patient under 138 lbs.
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2°C to 8°C (36° to 46°F). DO NOT FREEZE. DO NOT SHAKE.
Carton contents (4 prefilled cartridges, 1 package insert with attached
Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains
Dry Natural Rubber.
Do not Reuse
CAUTION, See package insert
for full prescribing information
and Instructions for Use
Rx Only
AMGEN®

PRINCIPAL DISPLAY PANEL - 50 mg/mL Cartridge Carton
Contains 4 Single-dose prefilled cartridges
NDC 58406-044-04
Enbrel®
etanercept
50
mg/mL
Not made with
natural rubber latex
Enbrel Mini® prefilled cartridge
50 mg/mL
Single-dose prefilled cartridge
For use with all AutoTouch® reusable autoinjectors only
Attention: Not for use in pediatric patient under 138 lbs.
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE. DO NOT SHAKE.
Carton contents (4 prefilled cartridges, 1 package insert with attached
Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
Do not Reuse
CAUTION, See package insert
for full prescribing information
and Instructions for Use
Rx Only
AMGEN®
↓ OPEN HERE ↓

PRINCIPAL DISPLAY PANEL
Contains 4 Single-dose prefilled Autoinjectors
NDC 58406-446-04
Enbrel®
etanercept
50 mg/mL
SureClick® 2.0 Autoinjector
Enhanced Features
see instructions for use
50 mg/mL
Single-dose Prefilled Autoinjector
Attention: Not for use in pediatric patients under 63 kg (138 pounds)
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Carton contents (4 prefilled SureClick® 2.0 Autoinjectors, 1 package insert with
attached Medication Guide) are intended to be dispensed as a unit.
ATTENTION: Enclosed Medication Guide is required for each patient.
This Product Contains Dry Natural Rubber.
AMGEN®
Rx Only
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320-1799
Marketed by Amgen Inc.

PRINCIPAL DISPLAY PANEL
NDC 58406-470-01
AMGEN®
AutoTouch® reusable autoinjector
For use with Enbrel Mini® (etanercept)
For use with Enbrel Mini® (etanercept) single-dose prefilled cartridge
Contains 1 AutoTouch® reusable autoinjector
For Subcutaneous Use Only
Store at room temperature.
IP52 – This package will resisit drops of water and dust.
Do Not Use if Package is Damaged
Type BF Applied Part
Exp: Expiry Date
Follow instructions for use
Rx Only

PRINCIPAL DISPLAY PANEL - 50 mg/mL Single-dose Prefilled Cartridge Autoinjector Label
NDC 58406-480-01
AMGEN®
Bluetooth®
AutoTouch Connect™
reusable autoinjector
For use with Enbrel Mini® (etanercept)
single-dose prefilled cartridge
Contains 1 AutoTouch Connect™ reusable autoinjector
For Subcutaneous Use Only
Store in a dry place at room temperature: 50°F to 104°F (10°C to 40°C)
IP52 – This package will resisit drops of water and dust.
Do Not Use if Package is Damaged
Type BF Applied Part
MR unsafe
Exp: Expiry Date
Follow instructions for use
Rx Only

PRINCIPAL DISPLAY PANEL - 25 mg/0.5 mL Vial Carton
4 Single-Dose Vials, each vial is 25 mg/0.5mL
NDC 58406-055-04
AMGEN®
Enbrel®
etanercept
Injection
25
mg
25 mg/0.5 mL
Single-Dose Vial – Discard unused portion
For Subcutaneous Use Only.
Sterile Solution - No Preservative.
Store refrigerated at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
DO NOT SHAKE.
Protect from Light.
U.S. License No. 1132
Manufactured by Immunex Corporation
Thousand Oaks, CA 91320-1799 U.S.A.
© 2020, 2022-2023 Immunex Corporation
Rx Only

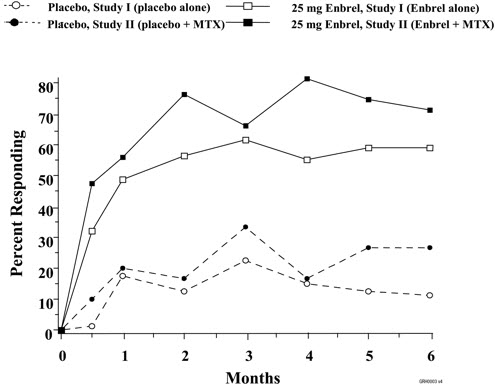
 This printed material is recyclable
This printed material is recyclable


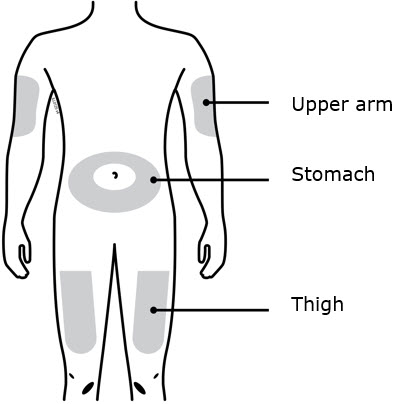
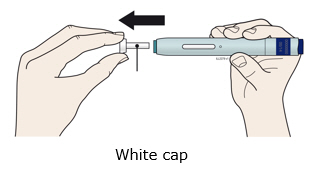
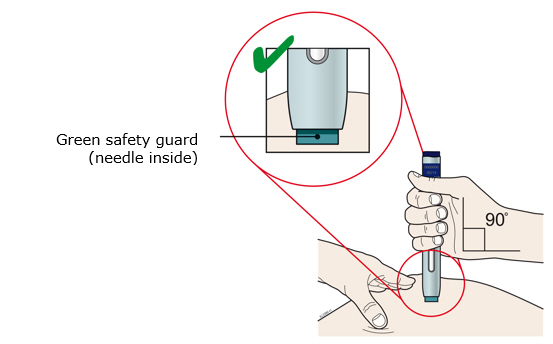
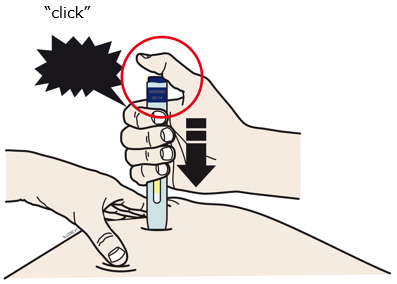
 ) to help guide your injection. When you receive a new autoinjector, it will be set with the sounds on (sound switch down).
) to help guide your injection. When you receive a new autoinjector, it will be set with the sounds on (sound switch down).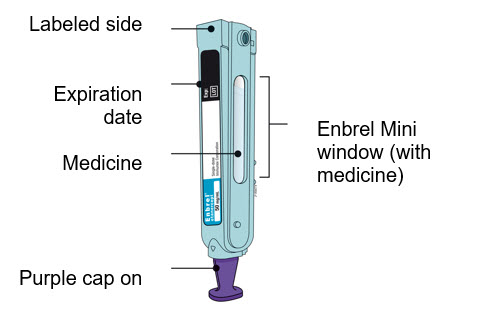
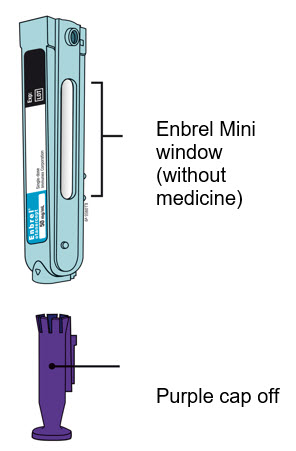
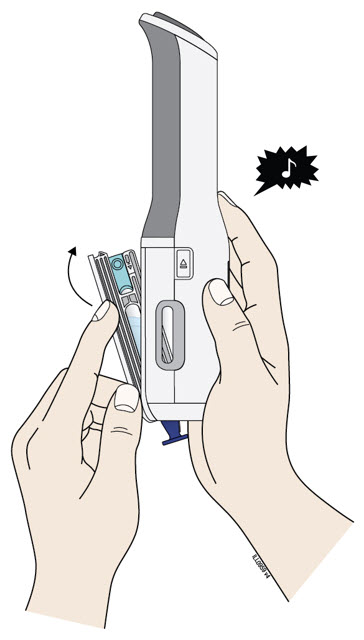

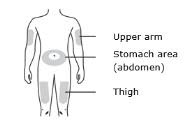
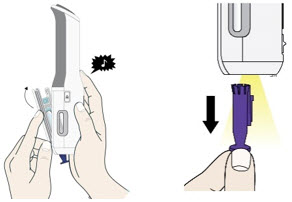









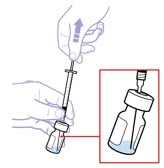
 This printed material is recyclable.
This printed material is recyclable.