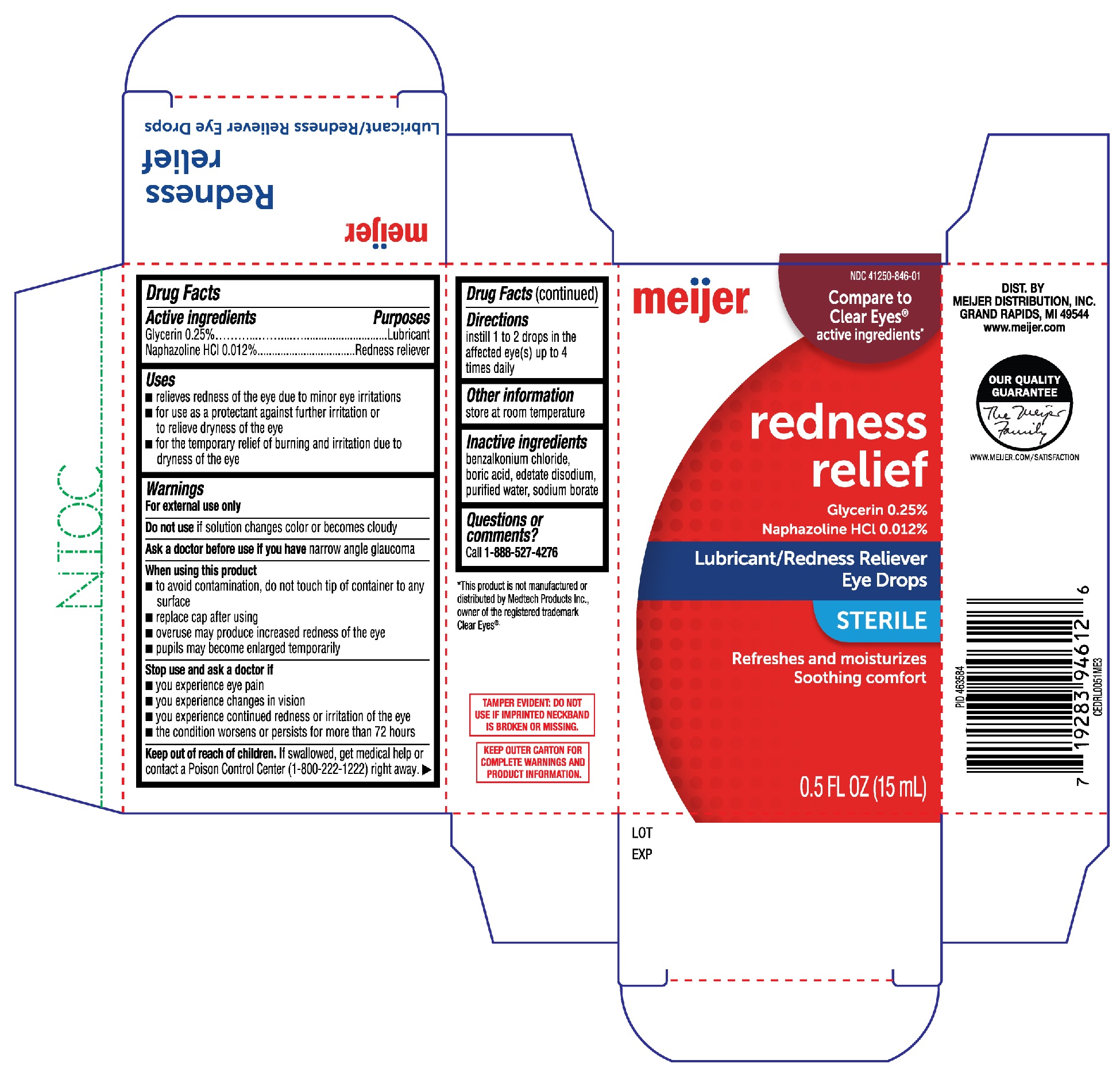
Active Ingredients
Glycerin 0.25%
Naphazoline HCI 0.012%
Purposes
Lubricant
Redness Reliever
Uses
- relieves redness of the eye due to minor eye irritations
- for use as a protectant against further irritation or to relieve dryness of the eye
- for the temporary relief of burning and irritation due to dryness of the eye
Warnings
For external use only
Do not use
if solution changes color or becomes cloudy
Ask a doctor before use if you have
narrow angle glaucoma
When using this product
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using
- overuse may produce increased redness of the eye
- pupils may become enlarged temporarily
Stop use and ask a doctor if
- you experience eye pain
- you experience changes in vision
- you experience continued redness or irriation of the eye
- the condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
instill 1 to 2 drops in the affected eye(s) up to 4 times daily
Other information
store at room temperature
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate
Questions or comments?
Call 1-888-527-4276
Meijer Redness Relief
Meijer Distribution, Inc.
