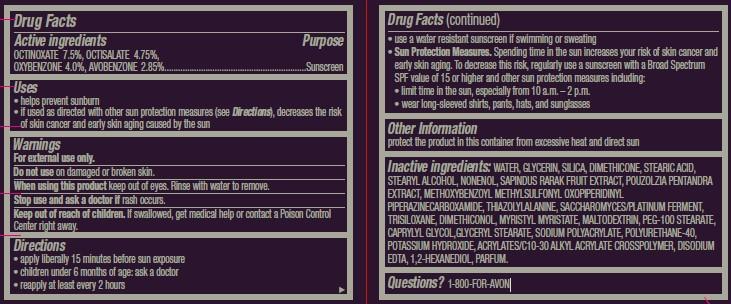
Active ingredients
OCTINOXATE 7.5%, OCTISALATE 4.75%,
OXYBENZONE 4.0%, AVOBENZONE 2.85%..................................
Uses
• helps prevent sunburn
• if used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
• apply liberally 15 minutes before sun exposure
• children under 6 months of age: ask a doctor
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
•
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
Inactive ingredients: WATER, GLYCERIN, SILICA, DIMETHICONE, STEARIC ACID, STEARYL ALCOHOL, NONENOL, SAPINDUS RARAK FRUIT EXTRACT, POUZOLZIA PENTANDRA EXTRACT, METHOXYBENZOYL METHYLSULFONYL OXOPIPERIDINYL PIPERAZINECARBOXAMIDE, THIAZOLYLALANINE, SACCHAROMYCES/PLATINUM FERMENT, TRISILOXANE, DIMETHICONOL, MYRISTYL MYRISTATE, MALTODEXTRIN, PEG-100 STEARATE, CAPRYLYL GLYCOL,GLYCERYL STEARATE, SODIUM POLYACRYLATE, POLYURETHANE-40, POTASSIUM HYDROXIDE, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, DISODIUM EDTA, 1,2-HEXANEDIOL, PARFUM.