FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Atorvastatin calcium tablets are indicated:
• To reduce the risk of:
o Myocardial infarction (MI), stroke, revascularization procedures, and angina in adults with multiple risk factors for coronary heart disease (CHD) but without clinically evident CHD
o MI and stroke in adults with type 2 diabetes mellitus with multiple risk factors for CHD but without clinically evident CHD
o Non-fatal MI, fatal and non-fatal stroke, revascularization procedures, hospitalization for congestive heart failure, and angina in adults with clinically evident CHD
• As an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) in:
o Adults with primary hyperlipidemia.
o Adults and pediatric patients aged 10 years and older with heterozygous familial hypercholesterolemia (HeFH).
• As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 10 years and older with homozygous familial hypercholesterolemia (HoFH).
• As an adjunct to diet for the treatment of adults with:
o Primary dysbetalipoproteinemia
o Hypertriglyceridemia
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
• Take atorvastatin calcium tablets orally once daily at any time of the day, with or without food.
• Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating atorvastatin calcium tablets, and adjust the dosage if necessary.
2.2 Recommended Dosage in Adult Patients
The recommended starting dosage of atorvastatin calcium tablets is 10 mg to 20 mg once daily. The dosage range is 10 mg to 80 mg once daily. Patients who require reduction in LDL-C greater than 45% may be started at 40 mg once daily.
2.3 Recommended Dosage in Pediatric Patients 10 Years of Age and Older with HeFH
The recommended starting dosage of atorvastatin calcium tablets is 10 mg once daily. The dosage range is 10 mg to 20 mg once daily.
2.4 Recommended Dosage in Pediatric Patients 10 Years of Age and Older with HoFH
The recommended starting dosage of atorvastatin calcium tablets is 10 mg to 20 mg once daily. The dosage range is 10 mg to 80 mg once daily.
2.5 Dosage Modifications Due to Drug Interactions
Concomitant use of atorvastatin calcium tablets with the following drugs requires dosage modification of atorvastatin calcium tablets [see Warnings and Precautions (5.1) and Drug Interactions (7.1)].
Anti-Viral Medications
• In patients taking saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir plus grazoprevir or letermovir, do not exceed atorvastatin calcium tablets 20 mg once daily.
• In patients taking nelfinavir, do not exceed atorvastatin calcium tablets 40 mg once daily.
Select Azole Antifungals or Macrolide Antibiotics
• In patients taking clarithromycin or itraconazole, do not exceed atorvastatin calcium tablets 20 mg once daily.
For additional recommendations regarding concomitant use of atorvastatin calcium tablets with other anti-viral medications, azole antifungals or macrolide antibiotics, see Drug Interactions (7.1)
3 DOSAGE FORMS AND STRENGTHS
Atorvastatin calcium tablets, USP:
10 mg atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with “LA37” on one side and plain on the other side.
20 mg of atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with “LA38” on one side and plain on the other side.
40 mg of atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with “LA39” on one side and plain on the other side.
80 mg of atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with “LA8” on one side and plain on the other side.
4 CONTRAINDICATIONS
• Acute liver failure or decompensated cirrhosis [see Warnings and Precautions (5.3)]
• Hypersensitivity to atorvastatin or any excipients in atorvastatin calcium tablets. Hypersensitivity reactions, including anaphylaxis, angioneurotic edema, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported [see Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy and Rhabdomyolysis
Atorvastatin may cause myopathy (muscle pain, tenderness, or weakness associated with elevated creatine kinase [CK]) and rhabdomyolysis. Acute kidney injury secondary to myoglobinuria and rare fatalities have occurred as a result of rhabdomyolysis in patients treated with statins, including atorvastatin.
Risk Factors for Myopathy
Risk factors for myopathy include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs (including other lipid-lowering therapies), and higher atorvastatin dosage [see Drug Interactions (7.1) and Use in Specific Populations (8.5, 8.6)].
Steps to Prevent or Reduce the Risk of Myopathy and Rhabdomyolysis
Atorvastatin exposure may be increased by drug interactions due to inhibition of cytochrome P450 enzyme 3A4 (CYP3A4) and/or transporters (e.g., breast cancer resistant protein [BCRP], organic anion-transporting polypeptide [OATP1B1/OATP1B3] and P-glycoprotein [P-gp]), resulting in an increased risk of myopathy and rhabdomyolysis. Concomitant use of cyclosporine, gemfibrozil, tipranavir plus ritonavir, or glecaprevir plus pibrentasvir with atorvastatin is not recommended. Atorvastatin dosage modifications are recommended for patients taking certain anti-viral, azole antifungals, or macrolide antibiotic medications [see Dosage and Administration (2.5)]. Cases of myopathy/rhabdomyolysis have been reported with atorvastatin co-administered with lipid modifying doses (>1 gram/day) of niacin, fibrates, colchicine, and ledipasvir plus sofosbuvir. Consider if the benefit of use of these products outweighs the increased risk of myopathy and rhabdomyolysis [see Drug Interactions (7.1)].
Concomitant intake of large quantities, more than 1.2 liters daily, of grapefruit juice is not recommended in patients taking atorvastatin [see Drug Interactions (7.1)].
Discontinue atorvastatin if markedly elevated CK levels occur or if myopathy is either diagnosed or suspected. Muscle symptoms and CK elevations may resolve if atorvastatin is discontinued. Temporarily discontinue atorvastatin in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis (e.g., sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy).
Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing the atorvastatin dosage. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
5.2 Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use, including reports of recurrence when the same or a different statin was administered. IMNM is characterized by proximal muscle weakness and elevated serum creatine kinase that persists despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Discontinue atorvastatin if IMNM is suspected.
5.3 Hepatic Dysfunction
Increases in serum transaminases have been reported with use of atorvastatin [see Adverse Reactions (6.1)]. In most cases, these changes appeared soon after initiation, were transient, were not accompanied by symptoms, and resolved or improved on continued therapy or after a brief interruption in therapy. Persistent increases to more than three times the ULN in serum transaminases have occurred in approximately 0.7% of patients receiving atorvastatin in clinical trials. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including atorvastatin.
Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury [see Use in Specific Populations (8.7)].
Consider liver enzyme testing before atorvastatin initiation and when clinically indicated thereafter. Atorvastatin is contraindicated in patients with acute liver failure or decompensated cirrhosis [see Contraindications (4)]. If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue atorvastatin.
5.4 Increases in HbA1c and Fasting Serum Glucose Levels
Increases in HbA1c and fasting serum glucose levels have been reported with statins, including atorvastatin. Optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices.
5.5 Increased Risk of Hemorrhagic Stroke in Patients on Atorvastatin 80 mg with Recent Hemorrhagic Stroke
In a post-hoc analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial where 2,365 adult patients, without CHD who had a stroke or TIA within the preceding 6 months, were treated with atorvastatin 80 mg, a higher incidence of hemorrhagic stroke was seen in the atorvastatin 80 mg group compared to placebo (55, 2.3% atorvastatin vs. 33, 1.4% placebo; HR: 1.68, 95% CI: 1.09, 2.59; p=0.0168). The incidence of fatal hemorrhagic stroke was similar across treatment groups (17 vs. 18 for the atorvastatin and placebo groups, respectively). The incidence of non-fatal hemorrhagic stroke was significantly higher in the atorvastatin group (38, 1.6%) as compared to the placebo group (16, 0.7%). Some baseline characteristics, including hemorrhagic and lacunar stroke on study entry, were associated with a higher incidence of hemorrhagic stroke in the atorvastatin group [see Adverse Reactions (6.1)]. Consider the risk/benefit of use of atorvastatin 80 mg in patients with recent hemorrhagic stroke.
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
• Myopathy and Rhabdomyolysis [see Warnings and Precautions (5.1)]
• Immune-Mediated Necrotizing Myopathy [see Warnings and Precautions (5.2)]
• Hepatic Dysfunction [see Warnings and Precautions (5.3)]
• Increases in HbA1c and Fasting Serum Glucose Levels [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the atorvastatin placebo-controlled clinical trial database of 16,066 patients (8,755 atorvastatin vs. 7,311 placebo; age range 10 to 93 years, 39% women, 91% White, 3% Black, 2% Asian, 4% other) with a median treatment duration of 53 weeks, the most common adverse reactions in patients treated with atorvastatin that led to treatment discontinuation and occurred at a rate greater than placebo were: myalgia (0.7%), diarrhea (0.5%), nausea (0.4%), alanine aminotransferase increase (0.4%), and hepatic enzyme increase (0.4%).
Table 1 summarizes adverse reactions reported in ≥ 2% and at a rate greater than placebo in patients treated with atorvastatin (n=8,755), from seventeen placebo-controlled trials.
| Adverse Reaction | % Placebo
N=7,311 | % 10 mg
N=3,908 | % 20 mg
N=188 | % 40 mg
N=604 | % 80 mg
N=4,055 | % Any dose
N=8,755 |
| Nasopharyngitis | 8.2 | 12.9 | 5.3 | 7.0 | 4.2 | 8.3 |
| Arthralgia | 6.5 | 8.9 | 11.7 | 10.6 | 4.3 | 6.9 |
| Diarrhea | 6.3 | 7.3 | 6.4 | 14.1 | 5.2 | 6.8 |
| Pain in extremity | 5.9 | 8.5 | 3.7 | 9.3 | 3.1 | 6.0 |
| Urinary tract infection | 5.6 | 6.9 | 6.4 | 8.0 | 4.1 | 5.7 |
| Dyspepsia | 4.3 | 5.9 | 3.2 | 6.0 | 3.3 | 4.7 |
| Nausea | 3.5 | 3.7 | 3.7 | 7.1 | 3.8 | 4.0 |
| Musculoskeletal pain | 3.6 | 5.2 | 3.2 | 5.1 | 2.3 | 3.8 |
| Muscle spasms | 3.0 | 4.6 | 4.8 | 5.1 | 2.4 | 3.6 |
| Myalgia | 3.1 | 3.6 | 5.9 | 8.4 | 2.7 | 3.5 |
| Insomnia | 2.9 | 2.8 | 1.1 | 5.3 | 2.8 | 3.0 |
| Pharyngolaryngeal pain | 2.1 | 3.9 | 1.6 | 2.8 | 0.7 | 2.3 |
Other adverse reactions reported in placebo-controlled trials include:
Body as a whole: malaise, pyrexia
Digestive system: abdominal discomfort, eructation, flatulence, hepatitis, cholestasis
Musculoskeletal system: musculoskeletal pain, muscle fatigue, neck pain, joint swelling
Metabolic and nutritional system: transaminases increase, liver function test abnormal, blood alkaline phosphatase increase, creatine phosphokinase increase, hyperglycemia
Nervous system: nightmare
Respiratory system: epistaxis
Skin and appendages: urticaria
Special senses: vision blurred, tinnitus
Urogenital system: white blood cells urine positive
Elevations in Liver Enzyme Tests
Persistent elevations in serum transaminases, defined as more than 3 times the ULN and occurring on 2 or more occasions, occurred in 0.7% of patients who received atorvastatin in clinical trials. The incidence of these abnormalities was 0.2%, 0.2%, 0.6%, and 2.3% for 10, 20, 40, and 80 mg, respectively.
One patient in clinical trials developed jaundice. Increases in liver enzyme tests in other patients were not associated with jaundice or other clinical signs or symptoms. Upon dose reduction, drug interruption, or discontinuation, transaminase levels returned to or near pretreatment levels without sequelae. Eighteen of 30 patients with persistent liver enzyme elevations continued treatment with a reduced dose of atorvastatin.
Treating to New Targets Study (TNT)
In TNT, [see Clinical Studies (14.1)] 10,001 patients (age range 29 to 78 years, 19% women; 94% White, 3% Black, 1% Asian, 2% other) with clinically evident CHD were treated with atorvastatin 10 mg daily (n=5,006) or atorvastatin 80 mg daily (n=4,995). In the high-dose atorvastatin group, there were more patients with serious adverse reactions (1.8%) and discontinuations due to adverse reactions (9.9%) as compared to the low-dose group (1.4%; 8.1%, respectively) during a median follow-up of 4.9 years. Persistent transaminase elevations (≥3 x ULN twice within 4 to 10 days) occurred in 1.3% of individuals with atorvastatin 80 mg and in 0.2% of individuals with atorvastatin 10 mg. Elevations of CK (≥ 10 x ULN) were higher in the high-dose atorvastatin group (0.3%) compared to the low-dose atorvastatin group (0.1%).
Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL)
In SPARCL, 4,731 patients (age range 21 to 92 years, 40% women; 93% White, 3% Black, 1% Asian, 3% other) without clinically evident CHD but with a stroke or transient ischemic attack (TIA) within the previous 6 months were treated with atorvastatin 80 mg (n=2,365) or placebo (n=2,366) for a median follow-up of 4.9 years. There was a higher incidence of persistent hepatic transaminase elevations (≥ 3 x ULN twice within 4 to 10 days) in the atorvastatin group (0.9%) compared to placebo (0.1%). Elevations of CK (>10 x ULN) were rare, but were higher in the atorvastatin group (0.1%) compared to placebo (0.0%). Diabetes was reported as an adverse reaction in 6.1% of subjects in the atorvastatin group and 3.8% of subjects in the placebo group.
In a post-hoc analysis, atorvastatin 80 mg reduced the incidence of ischemic stroke (9.2% vs. 11.6%) and increased the incidence of hemorrhagic stroke (2.3% vs. 1.4%) compared to placebo. The incidence of fatal hemorrhagic stroke was similar between groups (17 atorvastatin vs. 18 placebo). The incidence of non-fatal hemorrhagic strokes was significantly greater in the atorvastatin group (38 non- fatal hemorrhagic strokes) as compared to the placebo group (16 non-fatal hemorrhagic strokes). Patients who entered the trial with a hemorrhagic stroke appeared to be at increased risk for hemorrhagic stroke (16% atorvastatin vs. 4% placebo).
Adverse Reactions from Clinical Studies of Atorvastatin in Pediatric Patients with HeFH
In a 26-week controlled study in pediatric patients with HeFH (ages 10 years to 17 years) (n=140, 31% female; 92% White, 1.6% Blacks, 1.6% Asians, 4.8% other), the safety and tolerability profile of atorvastatin 10 to 20 mg daily, as an adjunct to diet to reduce total cholesterol, LDL-C, and apo B levels, was generally similar to that of placebo [see Use in Specific Populations (8.4) and Clinical Studies (14.6)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of atorvastatin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: pancreatitis
General disorders: fatigue
Hepatobiliary Disorders: fatal and non-fatal hepatic failure
Immune system disorders: anaphylaxis
Injury: tendon rupture
Musculoskeletal and connective tissue disorders: rhabdomyolysis, myositis.
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use.
Nervous system disorders: dizziness, peripheral neuropathy.
There have been rare reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with the use of all statins. Cognitive impairment was generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
Psychiatric disorders: depression
Respiratory disorders: interstitial lung disease
Skin and subcutaneous tissue disorders: angioneurotic edema, bullous rashes (including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis)
7 DRUG INTERACTIONS
7.1 Drug Interactions that may Increase the Risk of Myopathy and Rhabdomyolysis with Atorvastatin
Atorvastatin is a substrate of CYP3A4 and transporters (e.g., OATP1B1/1B3, P-gp, or BCRP). Atorvastatin plasma levels can be significantly increased with concomitant administration of inhibitors of CYP3A4 and transporters. Table 2 includes a list of drugs that may increase exposure to atorvastatin and may increase the risk of myopathy and rhabdomyolysis when used concomitantly and instructions for preventing or managing them [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
| Cyclosporine or Gemfibrozil
|
|
| Clinical Impact:
| Atorvastatin plasma levels were significantly increased with concomitant administration of atorvastatin and cyclosporine, an inhibitor of CYP3A4 and OATP1B1 [see Clinical Pharmacology (12.3)]. Gemfibrozil may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of cyclosporine or gemfibrozil with atorvastatin. |
| Intervention:
| Concomitant use of cyclosporine or gemfibrozil with atorvastatin is not recommended. |
| Anti-Viral Medications
|
|
| Clinical Impact:
| Atorvastatin plasma levels were significantly increased with concomitant administration of atorvastatin with many anti-viral medications, which are inhibitors of CYP3A4 and/or transporters (e.g., BCRP, OATP1B1/1B3, P-gp, MRP2, and/or OAT2) [see Clinical Pharmacology (12.3)]. Cases of myopathy and rhabdomyolysis have been reported with concomitant use of ledipasvir plus sofosbuvir with atorvastatin. |
| Intervention:
|
|
| Examples:
| Tipranavir plus ritonavir, glecaprevir plus pibrentasvir, lopinavir plus ritonavir, simeprevir, saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir plus grazoprevir, letermovir, nelfinavir, and ledipasvir plus sofosbuvir. |
| Select Azole Antifungals or Macrolide Antibiotics
|
|
| Clinical Impact:
| Atorvastatin plasma levels were significantly increased with concomitant administration of atorvastatin with select azole antifungals or macrolide antibiotics, due to inhibition of CYP3A4 and/or transporters [see Clinical Pharmacology (12.3)].
|
| Intervention:
| In patients taking clarithromycin or itraconazole, do not exceed atorvastatin 20 mg [see Dosage and Administration (2.5)]. Consider the risk/benefit of concomitant use of other azole antifungals or macrolide antibiotics with atorvastatin. Monitor all patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
| Examples:
| Erythromycin, clarithromycin, itraconazole, ketoconazole, posaconazole, and voriconazole. |
| Niacin
|
|
| Clinical Impact:
| Cases of myopathy and rhabdomyolysis have been observed with concomitant use of lipid modifying dosages of niacin (≥1 gram/day niacin) with atorvastatin. |
| Intervention:
| Consider if the benefit of using lipid modifying dosages of niacin concomitantly with atorvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
| Fibrates (other than Gemfibrozil)
|
|
| Clinical Impact:
| Fibrates may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of fibrates with atorvastatin. |
| Intervention:
| Consider if the benefit of using fibrates concomitantly with atorvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
| Colchicine
|
|
| Clinical Impact:
| Cases of myopathy and rhabdomyolysis have been reported with concomitant use of colchicine with atorvastatin. |
| Intervention:
| Consider the risk/benefit of concomitant use of colchicine with atorvastatin. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
| Grapefruit Juice
|
|
| Clinical Impact:
| Grapefruit juice consumption, especially excessive consumption, more than 1.2 liters/daily, can raise the plasma levels of atorvastatin and may increase the risk of myopathy and rhabdomyolysis. |
| Intervention:
| Avoid intake of large quantities of grapefruit juice, more than 1.2 liters daily, when taking atorvastatin. |
7.2 Drug Interactions that may Decrease Exposure to Atorvastatin
Table 3 presents drug interactions that may decrease exposure to atorvastatin and instructions for preventing or managing them.
| Rifampin
|
|
|
Clinical Impact: | Concomitant administration of atorvastatin with rifampin, an inducer of cytochrome P450 3A4 and inhibitor of OATP1B1, can lead to variable reductions in plasma concentrations of atorvastatin. Due to the dual interaction mechanism of rifampin, delayed administration of atorvastatin after administration of rifampin has been associated with a significant reduction in atorvastatin plasma concentrations. |
| Intervention:
| Administer atorvastatin and rifampin simultaneously. |
7.3 Atorvastatin Effects on Other Drugs
Table 4 presents atorvastatin’s effect on other drugs and instructions for preventing or managing them.
| Oral Contraceptives
|
|
| Clinical Impact:
| Co-administration of atorvastatin and an oral contraceptive increased plasma concentrations of norethindrone and ethinyl estradiol [see Clinical Pharmacology (12.3)].
|
| Intervention:
| Consider this when selecting an oral contraceptive for patients taking atorvastatin. |
| Digoxin
|
|
| Clinical Impact:
| When multiple doses of atorvastatin and digoxin were co-administered, steady state plasma digoxin concentrations increased [see Clinical Pharmacology (12.3)].
|
| Intervention:
| Monitor patients taking digoxin appropriately. |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Discontinue atorvastatin when pregnancy is recognized. Alternatively, consider the ongoing therapeutic needs of the individual patient. Atorvastatin decreases synthesis of cholesterol and possibly other biologically active substances derived from cholesterol; therefore, atorvastatin may cause fetal harm when administered to pregnant patients based on the mechanism of action [see Clinical Pharmacology (12.1)]. In addition, treatment of hyperlipidemia is not generally necessary during pregnancy. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hyperlipidemia for most patients.
Available data from case series and prospective and retrospective observational cohort studies over decades of use with statins in pregnant women have not identified a drug-associated risk of major congenital malformations. Published data from prospective and retrospective observational cohort studies with atorvastatin use in pregnant women are insufficient to determine if there is a drug- associated risk of miscarriage (see Data). In animal reproduction studies, no adverse developmental effects were observed in pregnant rats or rabbits orally administered atorvastatin at doses that resulted in up to 30 and 20 times, respectively, the human exposure at the maximum recommended human dose (MRHD) of 80 mg, based on body surface area (mg/m2). In rats administered atorvastatin during gestation and lactation, decreased postnatal growth and development delay were observed at doses ≥ 6 times the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
A Medicaid cohort linkage study of 1,152 statin-exposed pregnant women compared to 886,996 controls did not find a significant teratogenic effect from maternal use of statins in the first trimester of pregnancy, after adjusting for potential confounders – including maternal age, diabetes mellitus, hypertension, obesity, and alcohol and tobacco use – using propensity score-based methods. The relative risk of congenital malformations between the group with statin use and the group with no statin use in the first trimester was 1.07 (95% confidence interval 0.85 to 1.37) after controlling for confounders, particularly pre-existing diabetes mellitus. There were also no statistically significant increases in any of the organ-specific malformations assessed after accounting for confounders. In the majority of pregnancies, statin treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. Study limitations include reliance on physician coding to define the presence of a malformation, lack of control for certain confounders such as body mass index, use of prescription dispensing as verification for the use of a statin, and lack of information on non-live births.
Animal Data
Atorvastatin was administered to pregnant rats and rabbits during organogenesis at oral doses up to 300 mg/kg/day and 100 mg/kg/day, respectively. Atorvastatin was not teratogenic in rats at doses up to 300 mg/kg/day or in rabbits at doses up to 100 mg/kg/day. These doses resulted in multiples of about 30 times (rat) or 20 times (rabbit) the human exposure at the MRHD based on surface area (mg/m2). In rats, the maternally toxic dose of 300 mg/kg resulted in increased post-implantation loss and decreased fetal body weight. At the maternally toxic doses of 50 and 100 mg/kg/day in rabbits, there was increased post-implantation loss, and at 100 mg/kg/day fetal body weights were decreased.
In a study in pregnant rats administered 20, 100, or 225 mg/kg/day from gestation day 7 through to lactation day 20 (weaning), there was decreased survival at birth, postnatal day 4, weaning, and post-weaning in pups of mothers dosed with 225 mg/kg/day, a dose at which maternal toxicity was observed. Pup body weight was decreased through postnatal day 21 at 100 mg/kg/day, and through postnatal day 91 at 225 mg/kg/day. Pup development was delayed (rotorod performance at 100 mg/kg/day and acoustic startle at 225 mg/kg/day; pinnae detachment and eye-opening at 225 mg/kg/day). These doses correspond to 6 times (100 mg/kg) and 22 times (225 mg/kg) the human exposure at the MRHD, based on AUC.
Atorvastatin crosses the rat placenta and reaches a level in fetal liver equivalent to that of maternal plasma.
8.2 Lactation
Risk Summary
There is no information about the presence of atorvastatin in human milk, the effects of the drug on the breastfed infant or the effects of the drug on milk production. However, it has been shown that another drug in this class passes into human milk. Studies in rats have shown that atorvastatin and/or its metabolites are present in the breast milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data). Statins, including atorvastatin, decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol and may cause harm to the breastfed infant.
Because of the potential for serious adverse reactions in a breastfed infant, based on the mechanism of action, advise patients that breastfeeding is not recommended during treatment with atorvastatin [see Use in Specific Populations (8.1), Clinical Pharmacology (12.1)].
Data
Following a single oral administration of 10 mg/kg of radioactive atorvastatin to lactating rats, the concentration of total radioactivity was determined. Atorvastatin and/or its metabolites were measured in the breast milk and pup plasma at a 2:1 ratio (milk:plasma).
8.4 Pediatric Use
The safety and effectiveness of atorvastatin as an adjunct to diet to reduce LDL-C have been established pediatric patients 10 years of age and older with HeFH. Use of atorvastatin for this indication is based on a double-blind, placebo-controlled clinical trial in 187 pediatric patients 10 years of age and older with HeFH. In this limited controlled trial, there was no significant effect on growth or sexual maturation in the boys or girls, or on menstrual cycle length in girls.
The safety and effectiveness of atorvastatin as an adjunct to other LDL-C-lowering therapies to reduce LDL-C have been established pediatric patients 10 years of age and older with HoFH. Use of atorvastatin for this indication is based on a trial without a concurrent control group in 8 pediatric patients 10 years of age and older with HoFH [see Clinical Studies (14)].
The safety and effectiveness of atorvastatin have not been established in pediatric patients younger than 10 years of age with HeFH or HoFH, or in pediatric patients with other types of hyperlipidemia (other than HeFH or HoFH).
8.5 Geriatric Use
Of the total number of atorvastatin-treated patients in clinical trials, 15,813 (40%) were ≥65 years old and 2,800 (7%) were ≥75 years old. No overall differences in safety or effectiveness were observed between these patients and younger patients.
Advanced age (≥65 years) is a risk factor for atorvastatin-associated myopathy and rhabdomyolysis. Dose selection for an elderly patient should be cautious, recognizing the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of myopathy. Monitor geriatric patients receiving atorvastatin for the increased risk of myopathy [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Renal impairment is a risk factor for myopathy and rhabdomyolysis. Monitor all patients with renal impairment for development of myopathy. Renal impairment does not affect the plasma concentrations of atorvastatin, therefore there is no dosage adjustment in patients with renal impairment [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
In patients with chronic alcoholic liver disease, plasma concentrations of atorvastatin are markedly increased. Cmax and AUC are each 4-fold greater in patients with Childs-Pugh A disease. Cmax and AUC are approximately 16-fold and 11-fold increased, respectively, in patients with Childs-Pugh B disease. Atorvastatin is contraindicated in patients with acute liver failure or decompensated cirrhosis [see Contraindications (4)].
10 OVERDOSAGE
No specific antidotes for atorvastatin are known. Contact Poison Control (1-800-222-1222) for latest recommendations. Due to extensive drug binding to plasma proteins, hemodialysis is not expected to significantly enhance atorvastatin clearance.
11 DESCRIPTION
Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase.
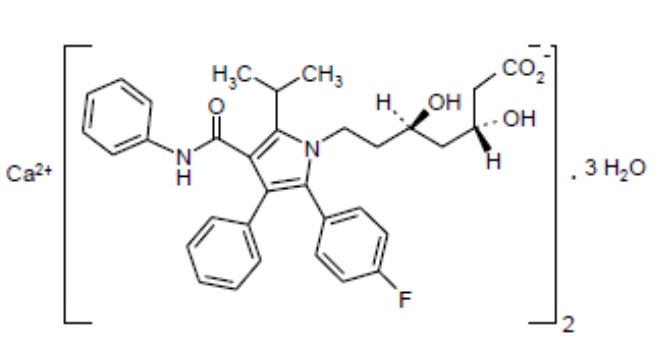
Atorvastatin calcium is calcium(βR,δR)-2-(p-fluorophenyl)-β,δ-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoate (1:2). The molecular formula of atorvastatin calcium is C66H68CaF2N4O10•3H2O and its molecular weight is 1,209.41. Its structural formula is:
Atorvastatin calcium USP is a white to off white powder that is insoluble in aqueous solutions of pH 4 and below. Atorvastatin calcium USP is insoluble to very slightly soluble in distilled water, in pH 7.4 phosphate buffer, and in acetonitrile; very slightly soluble to soluble in alcohol (99.9%); and soluble to freely soluble in methanol.
Atorvastatin calcium tablets, USP for oral use contain atorvastatin 10 mg, 20 mg, 40 mg, or 80 mg (equivalent to 10.36 mg, 20.72 mg, 41.44 mg, or 82.88 mg atorvastatin calcium anhydrous) and the following inactive ingredients: calcium carbonate, croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, Opadry White YS-1-7040 (hypromellose, polyethylene glycol, talc, titanium dioxide) and polysorbate 80.
Meets USP Dissolution Test 3.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atorvastatin is a selective, competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate, a precursor of sterols, including cholesterol. In animal models, atorvastatin lowers plasma cholesterol and lipoprotein levels by inhibiting HMG-CoA reductase and cholesterol synthesis in the liver and by increasing the number of hepatic LDL receptors on the cell surface to enhance uptake and catabolism of LDL; atorvastatin also reduces LDL production and the number of LDL particles.
12.2 Pharmacodynamics
Atorvastatin, as well as some of its metabolites, are pharmacologically active in humans. The liver is the primary site of action and the principal site of cholesterol synthesis and LDL clearance. Drug dosage, rather than systemic drug concentration, correlates better with LDL-C reduction. Individualization of drug dosage should be based on therapeutic response [see Dosage and Administration (2)].
12.3 Pharmacokinetics
Absorption
Atorvastatin is rapidly absorbed after oral administration; maximum plasma concentrations occur within 1 to 2 hours. Extent of absorption increases in proportion to atorvastatin dose. The absolute bioavailability of atorvastatin (parent drug) is approximately 14% and the systemic availability of HMG-CoA reductase inhibitory activity is approximately 30%. The low systemic availability is attributed to presystemic clearance in gastrointestinal mucosa and/or hepatic first-pass metabolism. Although food decreases the rate and extent of drug absorption by approximately 25% and 9%, respectively, as assessed by Cmax and AUC, LDL-C reduction is similar whether atorvastatin is given with or without food. Plasma atorvastatin concentrations are lower (approximately 30% for Cmax and AUC) following evening drug administration compared with morning. However, LDL-C reduction is the same regardless of the time of day of drug administration.
Distribution
Mean volume of distribution of atorvastatin is approximately 381 liters. Atorvastatin is ≥98% bound to plasma proteins. A blood/plasma ratio of approximately 0.25 indicates poor drug penetration into red blood cells.
Elimination
Metabolism
Atorvastatin is extensively metabolized to ortho- and parahydroxylated derivatives and various beta-oxidation products. In vitro inhibition of HMG-CoA reductase by ortho- and parahydroxylated metabolites is equivalent to that of atorvastatin. Approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites. In vitro studies suggest the importance of atorvastatin metabolism by cytochrome P450 3A4, consistent with increased plasma concentrations of atorvastatin in humans following co-administration with erythromycin, a known inhibitor of this isozyme [see Drug Interactions (7.1)]. In animals, the ortho-hydroxy metabolite undergoes further glucuronidation.
Excretion
Atorvastatin and its metabolites are eliminated primarily in bile following hepatic and/or extra-hepatic metabolism; however, the drug does not appear to undergo enterohepatic recirculation. Mean plasma elimination half-life of atorvastatin in humans is approximately 14 hours, but the half-life of inhibitory activity for HMG-CoA reductase is 20 to 30 hours due to the contribution of active metabolites. Less than 2% of a dose of atorvastatin is recovered in urine following oral administration.
Specific Populations
Geriatric
Plasma concentrations of atorvastatin are higher (approximately 40% for Cmax and 30% for AUC) in healthy elderly subjects (age ≥65 years) than in young adults.
Pediatric
Apparent oral clearance of atorvastatin in pediatric subjects appeared similar to that of adults when scaled allometrically by body weight as the body weight was the only significant covariate in atorvastatin population PK model with data including pediatric HeFH patients (ages 10 years to 17 years of age, n=29) in an open-label, 8-week study.
Gender
Plasma concentrations of atorvastatin in women differ from those in men (approximately 20% higher for Cmax and 10% lower for AUC); however, there is no clinically significant difference in LDL-C reduction with atorvastatin between men and women.
Renal Impairment
Renal disease has no influence on the plasma concentrations or LDL-C reduction of atorvastatin [see Use in Specific Populations (8.6)].
While studies have not been conducted in patients with end-stage renal disease, hemodialysis is not expected to significantly enhance clearance of atorvastatin since the drug is extensively bound to plasma proteins.
Hepatic Impairment
In patients with chronic alcoholic liver disease, plasma concentrations of atorvastatin are markedly increased. Cmax and AUC are each 4-fold greater in patients with Childs-Pugh A disease. Cmax and AUC are approximately 16-fold and 11-fold increased, respectively, in patients with Childs-Pugh B disease [see Use in Specific Populations (8.7)].
Drug Interactions
Atorvastatin is a substrate of the hepatic transporters, OATP1B1 and OATP1B3 transporter. Metabolites of atorvastatin are substrates of OATP1B1. Atorvastatin is also identified as a substrate of the efflux transporter BCRP, which may limit the intestinal absorption and biliary clearance of atorvastatin.
| Co-administered drug and
dosing regimen | Atorvastatin
|
||
| | Dose (mg)
| Ratio of AUC&
| Ratio of Cmax&
|
| #Cyclosporine 5.2 mg/kg/day, stable dose | 10 mg QDa for 28 days | 8.69 | 10.66 |
| #Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 10 mg SDc
| 9.36 | 8.58 |
| #Glecaprevir 400 mg QDa/pibrentasvir 120 mg QDa, 7 days | 10 mg QDa for 7 days | 8.28 | 22.00 |
| #Telaprevir 750 mg q8hf, 10 days | 20 mg SDc
| 7.88 | 10.60 |
| #, ‡Saquinavir 400 mg BIDb/ritonavir 400 mg BIDb, 15 days | 40 mg QDa for 4 days | 3.93 | 4.31 |
| #Elbasvir 50 mg QDa/grazoprevir 200 mg QDa, 13 days | 10 mg SDc
| 1.94 | 4.34 |
| #Simeprevir 150 mg QDa, 10 days | 40 mg SDc
| 2.12 | 1.70 |
| #Clarithromycin 500 mg BIDb, 9 days | 80 mg QDa for 8 days | 4.54 | 5.38 |
| #Darunavir 300 mg BIDb/ritonavir 100 mg BIDb, 9 days | 10 mg QDa for 4 days | 3.45 | 2.25 |
| #Itraconazole 200 mg QDa, 4 days | 40 mg SDc
| 3.32 | 1.20 |
| #Letermovir 480 mg QDa, 10 days | 20 mg SDc
| 3.29 | 2.17 |
| #Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 10 mg QDa for 4 days | 2.53 | 2.84 |
| #Fosamprenavir 1,400 mg BIDb, 14 days | 10 mg QDa for 4 days | 2.30 | 4.04 |
| #Nelfinavir 1,250 mg BIDb, 14 days | 10 mg QDa for 28 days | 1.74 | 2.22 |
| #Grapefruit Juice, 240 mL QDa,*
| 40 mg SDc
| 1.37 | 1.16 |
| Diltiazem 240 mg QDa, 28 days | 40 mg SDc
| 1.51 | 1.00 |
| Erythromycin 500 mg QIDe, 7 days | 10 mg SDc
| 1.33 | 1.38 |
| Amlodipine 10 mg, single dose | 80 mg SDc
| 1.18 | 0.91 |
| Cimetidine 300 mg QIDe, 2 weeks | 10 mg QDa for 2 weeks | 1.00 | 0.89 |
| Colestipol 10 g BIDb, 24 weeks | 40 mg QDa for 8 weeks | NA | 0.74**
|
| Maalox TC® 30 mL QIDe, 17 days | 10 mg QDa for 15 days | 0.66 | 0.67 |
| Efavirenz 600 mg QDa, 14 days | 10 mg for 3 days | 0.59 | 1.01 |
| #Rifampin 600 mg QDa, 7 days (co-administered)†
| 40 mg SDc
| 1.12 | 2.90 |
| #Rifampin 600 mg QDa, 5 days (doses separated)†
| 40 mg SDc
| 0.20 | 0.60 |
| #Gemfibrozil 600 mg BIDb, 7 days | 40 mg SDc
| 1.35 | 1.00 |
| #Fenofibrate 160 mg QDa, 7 days | 40 mg SDc
| 1.03 | 1.02 |
| Boceprevir 800 mg TIDd, 7 days | 40 mg SDc
| 2.32 | 2.66 |
& Represents ratio of treatments (co-administered drug plus atorvastatin vs. atorvastatin alone).
# See Sections 5.1 and 7 for clinical significance.
* Greater increases in AUC (ratio of AUC up to 2.5) and/or Cmax (ratio of Cmax up to 1.71) have been reported with excessive grapefruit consumption (≥ 750 mL-1.2 liters per day).
** Ratio based on a single sample taken 8 to 16 h post dose.
† Due to the dual interaction mechanism of rifampin, simultaneous co-administration of atorvastatin with rifampin is recommended, as delayed administration of atorvastatin after administration of rifampin has been associated with a significant reduction in atorvastatin plasma concentrations.
‡ The dose of saquinavir plus ritonavir in this study is not the clinically used dose. The increase in atorvastatin exposure when used clinically is likely to be higher than what was observed in this study. Therefore, caution should be applied and the lowest dose necessary should be used.
a Once daily
b Twice daily
c Single dose
d Three times daily
e Four times daily
f Every 8 hours
| Atorvastatin
| Co-administered drug and dosing regimen
|
||
| | Drug/Dose (mg)
| Ratio of AUC
| Ratio of Cmax
|
| 80 mg QDa for 15 days | Antipyrine, 600 mg SDc
| 1.03 | 0.89 |
| 80 mg QDa for 10 days | # Digoxin 0.25 mg QDa, 20 days | 1.15 | 1.20 |
|
40 mg QDa for 22 days | Oral contraceptive QDa, 2 months - norethindrone 1 mg - ethinyl estradiol 35 mcg |
1.28 1.19 |
1.23 1.30 |
| 10 mg SDc
| Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 1.08 | 0.96 |
| 10 mg QDa for 4 days | Fosamprenavir 1,400 mg BIDb, 14 days | 0.73 | 0.82 |
| 10 mg QDa for 4 days | Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 0.99 | 0.94 |
# See Section 7 for clinical significance.
a Once daily
b Twice daily
c Single dose
Atorvastatin had no clinically significant effect on prothrombin time when administered to patients receiving chronic warfarin treatment.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in rats at dose levels of 10, 30, and 100 mg/kg/day, 2 rare tumors were found in muscle in high-dose females: in one, there was a rhabdomyosarcoma and, in another, there was a fibrosarcoma. This dose represents a plasma AUC (0 to 24) value of approximately 16 times the mean human plasma drug exposure after an 80 mg oral dose.
A 2-year carcinogenicity study in mice given 100, 200, or 400 mg/kg/day resulted in a significant increase in liver adenomas in high-dose males and liver carcinomas in high-dose females. These findings occurred at plasma AUC (0 to 24) values of approximately 6 times the mean human plasma drug exposure after an 80 mg oral dose.
In vitro, atorvastatin was not mutagenic or clastogenic in the following tests with and without metabolic activation: the Ames test with Salmonella typhimurium and Escherichia coli, the HGPRT forward mutation assay in Chinese hamster lung cells, and the chromosomal aberration assay in Chinese hamster lung cells. Atorvastatin was negative in the in vivo mouse micronucleus test.
In female rats, atorvastatin at doses up to 225 mg/kg (56 times the human exposure) did not cause adverse effects on fertility. Studies in male rats performed at doses up to 175 mg/kg (15 times the human exposure) produced no changes in fertility. There was aplasia and aspermia in the epididymis of 2 of 10 rats treated with 100 mg/kg/day of atorvastatin for 3 months (16 times the human AUC at the 80 mg dose); testis weights were significantly lower at 30 and 100 mg/kg and epididymal weight was lower at 100 mg/kg. Male rats given 100 mg/kg/day for 11 weeks prior to mating had decreased sperm motility, spermatid head concentration, and increased abnormal sperm. Atorvastatin caused no adverse effects on semen parameters, or reproductive organ histopathology in dogs given doses of 10, 40, or 120 mg/kg for 2 years.
14 CLINICAL STUDIES
Prevention of Cardiovascular Disease
In the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), the effect of atorvastatin on fatal and non-fatal coronary heart disease was assessed in 10,305 patients with hypertension, 40 to 80 years of age (mean of 63 years; 19% women; 95% White, 3% Black, 1% South Asian, 1% other), without a previous myocardial infarction and with total cholesterol (TC) levels ≤251 mg/dL. Additionally, all patients had at least 3 of the following cardiovascular risk factors: male gender (81%), age >55 years (85%), smoking (33%), diabetes (24%), history of CHD in a first-degree relative (26%), TC:HDL >6 (14%), peripheral vascular disease (5%), left ventricular hypertrophy (14%), prior cerebrovascular event (10%), specific ECG abnormality (14%), proteinuria/albuminuria (62%). In this double-blind, placebo-controlled trial, patients were treated with anti-hypertensive therapy (goal BP <140/90 mm Hg for patients without diabetes; <130/80 mm Hg for patients with diabetes) and allocated to either atorvastatin 10 mg daily (n=5,168) or placebo (n=5,137), using a covariate adaptive method which took into account the distribution of nine baseline characteristics of patients already enrolled and minimized the imbalance of those characteristics across the groups. Patients were followed for a median duration of 3.3 years.
The effect of 10 mg/day of atorvastatin on lipid levels was similar to that seen in previous clinical trials.
Atorvastatin significantly reduced the rate of coronary events [either fatal coronary heart disease (46 events in the placebo group vs. 40 events in the atorvastatin group) or non-fatal MI (108 events in the placebo group vs. 60 events in the atorvastatin group)] with a relative risk reduction of 36% [(based on incidences of 1.9% for atorvastatin vs. 3.0% for placebo), p=0.0005 (see Figure 1)]. The risk reduction was consistent regardless of age, smoking status, obesity, or presence of renal dysfunction. The effect of atorvastatin was seen regardless of baseline LDL levels.
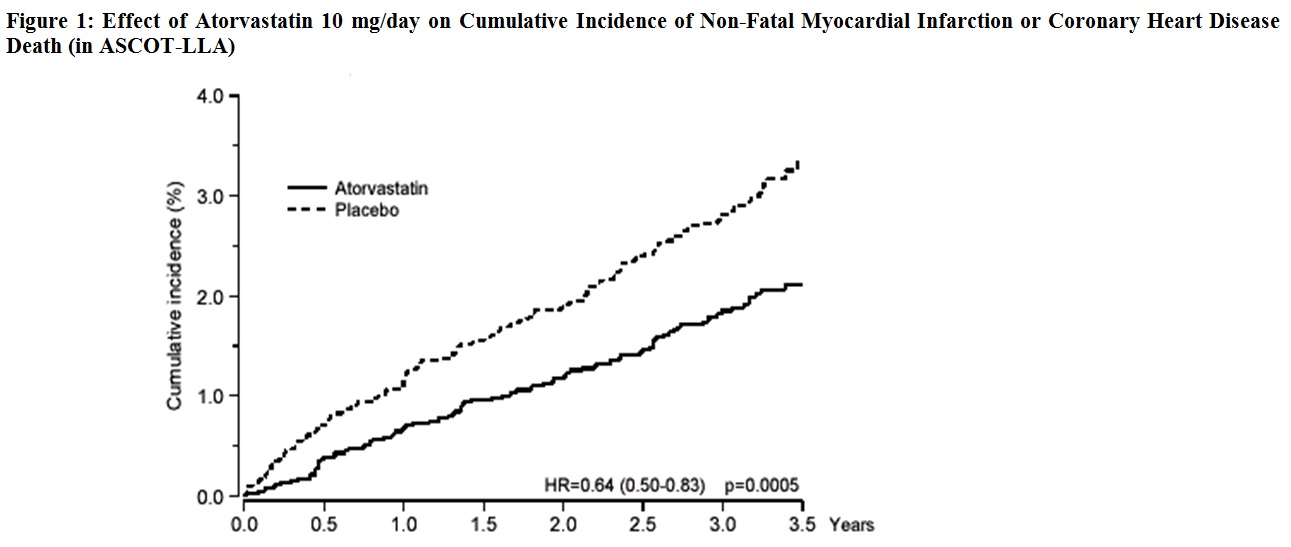
Atorvastatin also significantly decreased the relative risk for revascularization procedures by 42% (incidences of 1.4% for atorvastatin and 2.5% for placebo). Although the reduction of fatal and non-fatal strokes did not reach a pre-defined significance level (p=0.01), a favorable trend was observed with a 26% relative risk reduction (incidences of 1.7% for atorvastatin and 2.3% for placebo). There was no significant difference between the treatment groups for death due to cardiovascular causes (p=0.51) or noncardiovascular causes (p=0.17).
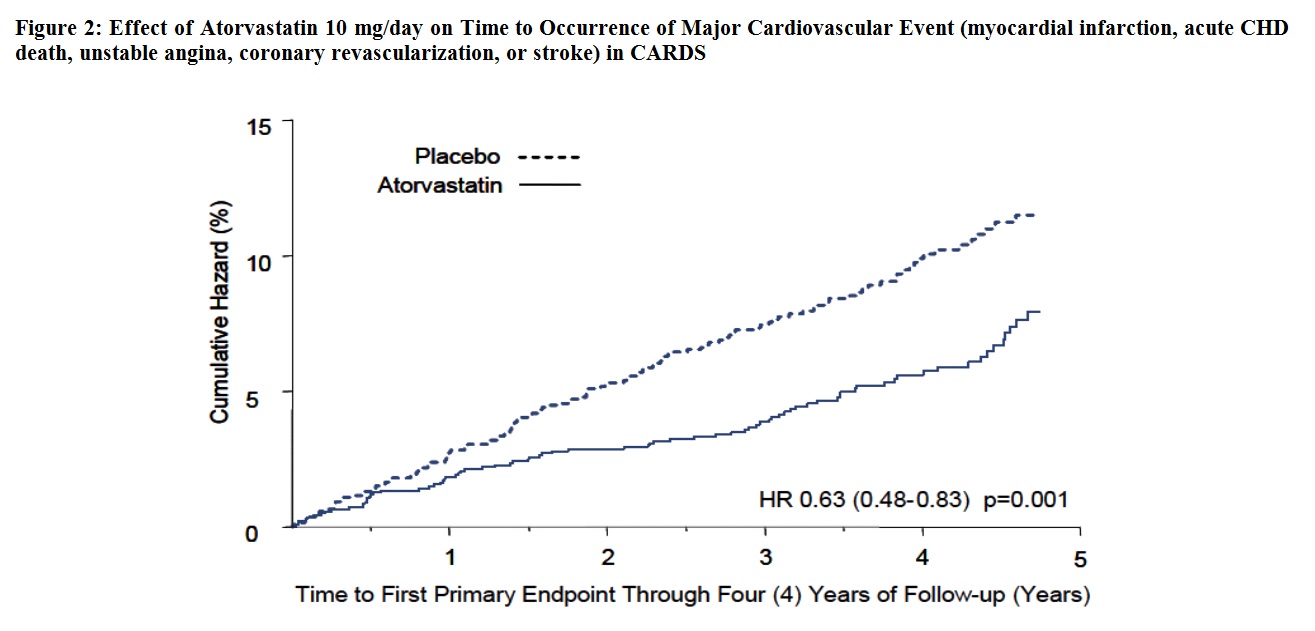
In the Collaborative Atorvastatin Diabetes Study (CARDS), the effect of atorvastatin on cardiovascular disease (CVD) endpoints was assessed in 2,838 subjects (94% White, 2% Black, 2% South Asian, 1% other; 68% male), ages 40 to 75 with type 2 diabetes based on WHO criteria, without prior history of cardiovascular disease and with LDL ≤160 mg/dL and triglycerides (TG) ≤600 mg/dL. In addition to diabetes, subjects had 1 or more of the following risk factors: current smoking (23%), hypertension (80%), retinopathy (30%), or microalbuminuria (9%) or macroalbuminuria (3%). No subjects on hemodialysis were enrolled in the trial. In this multicenter, placebo-controlled, double-blind clinical trial, subjects were randomly allocated to either atorvastatin 10 mg daily (1,429) or placebo (1,411) in a 1:1 ratio and were followed for a median duration of 3.9 years. The primary endpoint was the occurrence of any of the major cardiovascular events: myocardial infarction, acute CHD death, unstable angina, coronary revascularization, or stroke. The primary analysis was the time to first occurrence of the primary endpoint.
Baseline characteristics of subjects were: mean age of 62 years, mean HbA1c 7.7%; median LDL-C 120 mg/dL; median TC 207 mg/dL; median TG 151 mg/dL; median HDL-C 52 mg/dL.
The effect of atorvastatin 10 mg/day on lipid levels was similar to that seen in previous clinical trials.
Atorvastatin significantly reduced the rate of major cardiovascular events (primary endpoint events) (83 events in the atorvastatin group vs. 127 events in the placebo group) with a relative risk reduction of 37%, HR 0.63, 95% CI (0.48, 0.83) (p=0.001) (see Figure 2). An effect of atorvastatin was seen regardless of age, sex, or baseline lipid levels.
Atorvastatin significantly reduced the risk of stroke by 48% (21 events in the atorvastatin group vs. 39 events in the placebo group), HR 0.52, 95% CI (0.31, 0.89) (p=0.016) and reduced the risk of MI by 42% (38 events in the atorvastatin group vs. 64 events in the placebo group), HR 0.58, 95.1% CI (0.39, 0.86) (p=0.007). There was no significant difference between the treatment groups for angina, revascularization procedures, and acute CHD death.
There were 61 deaths in the atorvastatin group vs. 82 deaths in the placebo group (HR 0.73, p=0.059).
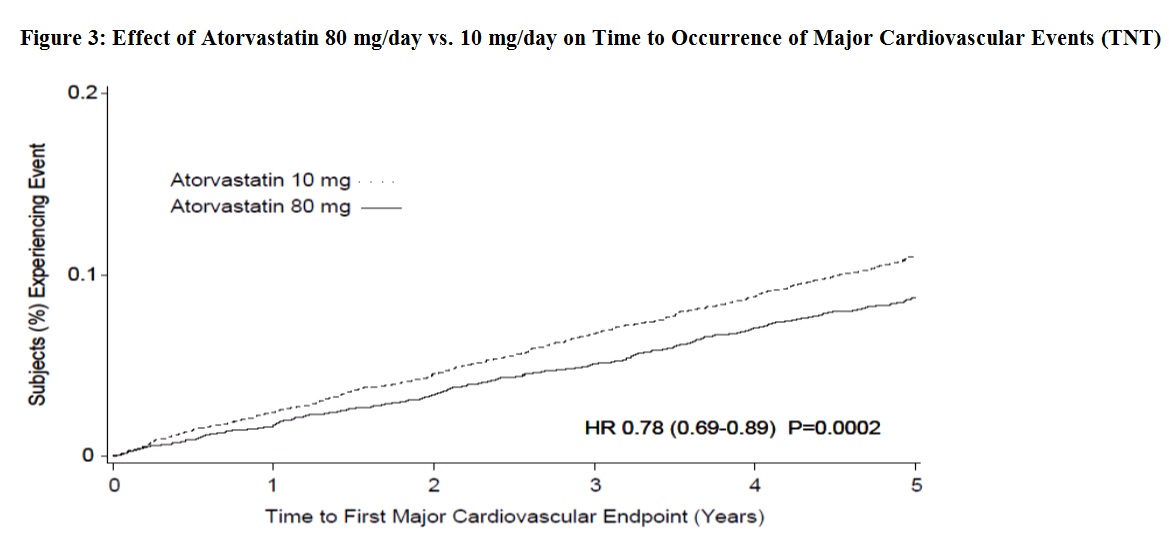
In the Treating to New Targets Study (TNT), the effect of atorvastatin 80 mg/day vs. atorvastatin 10 mg/day on the reduction in cardiovascular events was assessed in 10,001 subjects (94% White, 81% male, 38% ≥65 years) with clinically evident coronary heart disease who had achieved a target LDL-C level <130 mg/dL after completing an 8-week, open-label, run-in period with atorvastatin 10 mg/day. Subjects were randomly assigned to either 10 mg/day or 80 mg/day of atorvastatin and followed for a median duration of 4.9 years. The primary endpoint was the time-to-first occurrence of any of the following major cardiovascular events (MCVE): death due to CHD, non-fatal myocardial infarction, resuscitated cardiac arrest, and fatal and non-fatal stroke. The mean LDL-C, TC, TG, non-HDL, and HDL cholesterol levels at 12 weeks were 73, 145, 128, 98, and 47 mg/dL during treatment with 80 mg of atorvastatin and 99, 177, 152, 129, and 48 mg/dL during treatment with 10 mg of atorvastatin.
Treatment with atorvastatin 80 mg/day significantly reduced the rate of MCVE (434 events in the 80 mg/day group vs. 548 events in the 10 mg/day group) with a relative risk reduction of 22%, HR 0.78, 95% CI (0.69, 0.89), p=0.0002 (see Figure 3 and Table 7). The overall risk reduction was consistent regardless of age (<65, ≥65) or sex.
|
a Atorvastatin 80 mg: atorvastatin 10 mg b Component of other secondary endpoints * Secondary endpoints not included in primary endpoint HR=hazard ratio; CHD=coronary heart disease; CI=confidence interval; MI=myocardial infarction; CHF=congestive heart failure; CV=cardiovascular; PVD=peripheral vascular disease; CABG=coronary artery bypass graft Confidence intervals for the Secondary Endpoints were not adjusted for multiple comparisons |
|||||
| Endpoint
| Atorvastatin 10 mg (N=5,006)
| Atorvastatin 80 mg (N=4,995)
| HRa (95%CI) |
||
| PRIMARY ENDPOINT
| n | (%) | n | (%) | |
| First major cardiovascular endpoint | 548 | (10.9) | 434 | (8.7) | 0.78 (0.69, 0.89) |
| Components of the Primary Endpoint
| | | | | |
| CHD death | 127 | (2.5) | 101 | (2.0) | 0.80 (0.61, 1.03) |
| Non-fatal, non-procedure related MI | 308 | (6.2) | 243 | (4.9) | 0.78 (0.66, 0.93) |
| Resuscitated cardiac arrest | 26 | (0.5) | 25 | (0.5) | 0.96 (0.56, 1.67) |
| Stroke (fatal and non-fatal) | 155 | (3.1) | 117 | (2.3) | 0.75 (0.59, 0.96) |
| SECONDARY ENDPOINTS*
| | | | | |
| First CHF with hospitalization | 164 | (3.3) | 122 | (2.4) | 0.74 (0.59, 0.94) |
| First PVD endpoint | 282 | (5.6) | 275 | (5.5) | 0.97 (0.83, 1.15) |
| First CABG or other coronary revascularization procedureb
| 904 | (18.1) | 667 | (13.4) | 0.72 (0.65, 0.80) |
| First documented angina endpointb
| 615 | (12.3) | 545 | (10.9) | 0.88 (0.79, 0.99) |
| All-cause mortality | 282 | (5.6) | 284 | (5.7) | 1.01 (0.85, 1.19) |
| Components of All-Cause Mortality
| | | | | |
| Cardiovascular death | 155 | (3.1) | 126 | (2.5) | 0.81 (0.64, 1.03) |
| Noncardiovascular death | 127 | (2.5) | 158 | (3.2) | 1.25 (0.99, 1.57) |
| Cancer death | 75 | (1.5) | 85 | (1.7) | 1.13 (0.83, 1.55) |
| Other non-CV death | 43 | (0.9) | 58 | (1.2) | 1.35 (0.91, 2.00) |
| Suicide, homicide, and other traumatic non-CV death | 9 | (0.2) | 15 | (0.3) | 1.67 (0.73, 3.82) |
Of the events that comprised the primary efficacy endpoint, treatment with atorvastatin 80 mg/day significantly reduced the rate of non-fatal, non-procedure related MI and fatal and non-fatal stroke, but not CHD death or resuscitated cardiac arrest (Table 7). Of the predefined secondary endpoints, treatment with atorvastatin 80 mg/day significantly reduced the rate of coronary revascularization, angina, and hospitalization for heart failure, but not peripheral vascular disease. The reduction in the rate of CHF with hospitalization was only observed in the 8% of patients with a prior history of CHF.
There was no significant difference between the treatment groups for all-cause mortality (Table 7). The proportions of subjects who experienced cardiovascular death, including the components of CHD death and fatal stroke, were numerically smaller in the atorvastatin 80 mg group than in the atorvastatin 10 mg treatment group. The proportions of subjects who experienced noncardiovascular death were numerically larger in the atorvastatin 80 mg group than in the atorvastatin 10 mg treatment group.
Primary Hyperlipidemia in Adults
Atorvastatin reduces total-C, LDL-C, apo B, and TG, and increases HDL-C in patients with hyperlipidemia (heterozygous familial and nonfamilial) and mixed dyslipidemia. Therapeutic response is seen within 2 weeks, and maximum response is usually achieved within 4 weeks and maintained during chronic therapy.
In two multicenter, placebo-controlled, dose-response trials in patients with hyperlipidemia, atorvastatin given as a single dose over 6 weeks, significantly reduced total-C, LDL-C, apo B, and TG. (Pooled results are provided in Table 8.)
|
a Results are pooled from 2 dose-response trials. |
||||||
| Dose | N | TC | LDL-C | Apo B | TG | HDL-C |
| Placebo | 21 | 4 | 4 | 3 | 10 | -3 |
| 10 | 22 | -29 | -39 | -32 | -19 | 6 |
| 20 | 20 | -33 | -43 | -35 | -26 | 9 |
| 40 | 21 | -37 | -50 | -42 | -29 | 6 |
| 80 | 23 | -45 | -60 | -50 | -37 | 5 |
In three multicenter, double-blind trials in patients with hyperlipidemia, atorvastatin was compared to other statins. After randomization, patients were treated for 16 weeks with either atorvastatin 10 mg per day or a fixed dose of the comparative agent (Table 9).
|
1 A negative value for the 95% CI for the difference between treatments favors atorvastatin for all except HDL-C, for which a positive value favors atorvastatin. If the range does not include 0, this indicates a statistically significant difference. a Significantly different from lovastatin, ANCOVA, p ≤0.05 b Significantly different from pravastatin, ANCOVA, p ≤0.05 c Significantly different from simvastatin, ANCOVA, p ≤0.05 |
||||||
| Treatment
(Daily Dose) | N | Total-C | LDL-C | Apo B | TG | HDL-C |
| Trial 1
| | | | | | |
| Atorvastatin 10 mg | 707 | -27a
| -36a
| -28a
| -17a
| +7 |
| Lovastatin 20 mg | 191 | -19 | -27 | -20 | -6 | +7 |
| 95% CI for Diff1
| | -9.2, -6.5 | -10.7, -7.1 | -10.0, -6.5 | -15.2, -7.1 | -1.7, 2.0 |
| Trial 2
| | | | | | |
| Atorvastatin 10 mg | 222 | -25b
| -35b
| -27b
| -17b
| +6 |
| Pravastatin 20 mg | 77 | -17 | -23 | -17 | -9 | +8 |
| 95% CI for Diff1
| | -10.8, -6.1 | -14.5, -8.2 | -13.4, -7.4 | -14.1, -0.7 | -4.9, 1.6 |
| Trial 3
| | | | | | |
| Atorvastatin 10 mg | 132 | -29c
| -37c
| -34c
| -23c
| +7 |
| Simvastatin 10 mg | 45 | -24 | -30 | -30 | -15 | +7 |
| 95% CI for Diff1
| | -8.7, -2.7 | -10.1, -2.6 | -8.0, -1.1 | -15.1, -0.7 | -4.3, 3.9 |
Table 9 does not contain data comparing the effects of atorvastatin 10 mg and higher doses of lovastatin, pravastatin, and simvastatin. The drugs compared in the trials summarized in the table are not necessarily interchangeable.
Hypertriglyceridemia in Adults
The response to atorvastatin in 64 patients with isolated hypertriglyceridemia treated across several clinical trials is shown in the table below (Table 10). For the atorvastatin-treated patients, median (min, max) baseline TG level was 565 (267 to 1,502).
| | Placebo (N=12) | Atorvastatin 10 mg (N=37) | Atorvastatin 20 mg (N=13) | Atorvastatin 80 mg (N=14) |
| Triglycerides | -12.4 (-36.6, 82.7) | -41.0 (-76.2, 49.4) | -38.7 (-62.7, 29.5) | -51.8 (-82.8, 41.3) |
| Total-C | -2.3 (-15.5, 24.4) | -28.2 (-44.9, -6.8) | -34.9 (-49.6, -15.2) | -44.4 (-63.5, -3.8) |
| LDL-C | 3.6 (-31.3, 31.6) | -26.5 (-57.7, 9.8) | -30.4 (-53.9, 0.3) | -40.5 (-60.6, -13.8) |
| HDL-C | 3.8 (-18.6, 13.4) | 13.8 (-9.7, 61.5) | 11.0 (-3.2, 25.2) | 7.5 (-10.8, 37.2) |
| non-HDL-C | -2.8 (-17.6, 30.0) | -33.0 (-52.1, -13.3) | -42.7 (-53.7, -17.4) | -51.5 (-72.9, -4.3) |
Dysbetalipoproteinemia in Adults
The results of an open-label crossover trial of 16 patients (genotypes: 14 apo E2/E2 and 2 apo E3/E2) with dysbetalipoproteinemia are shown in the table below (Table 11).
| | | Median % Change (min, max) |
|
| | Median (min, max) at Baseline (mg/dL) | Atorvastatin 10 mg | Atorvastatin 80 mg |
| Total-C | 442 (225, 1,320) | -37 (-85, 17) | -58 (-90, -31) |
| Triglycerides | 678 (273, 5,990) | -39 (-92, -8) | -53 (-95, -30) |
| IDL-C + VLDL-C | 215 (111, 613) | -32 (-76, 9) | -63 (-90, -8) |
| non-HDL-C | 411 (218, 1,272) | -43 (-87, -19) | -64 (-92, -36) |
HoFH in Adults and Pediatric Patients
In a trial without a concurrent control group, 29 patients (mean age of 22 years, median age of 24 years, 31% <18 years) with HoFH received maximum daily doses of 20 to 80 mg of atorvastatin. The mean LDL-C reduction in this trial was 18%. Twenty-five patients with a reduction in LDL-C had a mean response of 20% (range of 7% to 53%, median of 24%); the remaining 4 patients had 7% to 24% increases in LDL-C. Five of the 29 patients had absent LDL-receptor function. Of these, 2 patients also had a portacaval shunt and had no significant reduction in LDL-C. The remaining 3 receptor-negative patients had a mean LDL-C reduction of 22%.
HeFH in Pediatric Patients
In a double-blind, placebo-controlled trial followed by an open-label phase, 187 boys and post-menarchal girls 10 years to 17 years of age (mean age 14.1 years; 31% female; 92% White, 1.6% Blacks, 1.6% Asians, 4.8% other) with heterozygous familial hypercholesterolemia (HeFH) or severe hypercholesterolemia, were randomized to atorvastatin (n=140) or placebo (n=47) for 26 weeks and then all received atorvastatin for 26 weeks. Inclusion in the trial required 1) a baseline LDL-C level ≥190 mg/dL or 2) a baseline LDL-C level ≥160 mg/dL and positive family history of FH or documented premature cardiovascular disease in a first or second-degree relative. The mean baseline LDL-C value was 219 mg/dL (range: 139 to 385 mg/dL) in the atorvastatin group compared to 230 mg/dL (range: 160 to 325 mg/dL) in the placebo group. The dosage of atorvastatin (once daily) was 10 mg for the first 4 weeks and uptitrated to 20 mg if the LDL-C level was >130 mg/dL. The number of atorvastatin-treated patients who required uptitration to 20 mg after Week 4 during the double-blind phase was 78 (56%).
Atorvastatin significantly decreased plasma levels of total-C, LDL-C, triglycerides, and apolipoprotein B during the 26-week double-blind phase (see Table 12).
| DOSAGE | N | Total-C | LDL-C | HDL-C | TG | Apolipoprotein B |
| Placebo | 47 | -1.5 | -0.4 | -1.9 | 1.0 | 0.7 |
| Atorvastatin | 140 | -31.4 | -39.6 | 2.8 | -12.0 | -34.0 |
The mean achieved LDL-C value was 130.7 mg/dL (range: 70 to 242 mg/dL) in the atorvastatin group compared to 228.5 mg/dL (range: 152 to 385 mg/dL) in the placebo group during the 26-week double-blind phase.
Atorvastatin was also studied in a three year open-label, uncontrolled trial that included 163 patients with HeFH who were 10 years to 15 years old (82 boys and 81 girls). All patients had a clinical diagnosis of HeFH confirmed by genetic analysis (if not already confirmed by family history). Approximately 98% were White, and less than 1% were Black or Asian. Mean LDL-C at baseline was 232 mg/dL. The starting atorvastatin dosage was 10 mg once daily and doses were adjusted to achieve a target of<130 mg/dL LDL-C. The reductions in LDL-C from baseline were generally consistent across age groups within the trial as well as with previous clinical trials in both adult and pediatric placebo-controlled trials.
16 HOW SUPPLIED/STORAGE AND HANDLING
Atorvastatin calcium tablets are supplied as follows:
10 mg atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with "LA37" on one side and plain on the other side.
Bottles of 30 NDC 42385-940-30
Bottles of 90 NDC 42385-940-90
Bottles of 100 NDC 42385-940-01
Bottles of 500 NDC 42385-940-05
Bottles of 1,000 NDC 42385-940-11
Carton with 100 (10 x 10) Unit-Dose Tablets NDC 42385-940-68
20 mg of atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with "LA38" on one side and plain on the other side.
Bottles of 30 NDC 42385-941-30
Bottles of 90 NDC 42385-941-90
Bottles of 100 NDC 42385-941-01
Bottles of 500 NDC 42385-941-05
Bottles of 1,000 NDC 42385-941-11
Carton with 100 (10 x 10) Unit-Dose Tablets NDC 42385-941-68
40 mg of atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with "LA39" on one side and plain on the other side.
Bottles of 30 NDC 42385-942-30
Bottles of 90 NDC 42385-942-90
Bottles of 100 NDC 42385-942-01
Bottles of 500 NDC 42385-942-05
Bottles of 1,000 NDC 42385-942-11
Carton with 100 (10 x 10) Unit-Dose Tablets NDC 42385-942-68
80 mg of atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with "LA8" on one side and plain on the other side.
Bottles of 30 NDC 42385-943-30
Bottles of 90 NDC 42385-943-90
Bottles of 100 NDC 42385-943-01
Bottles of 500 NDC 42385-943-05
Bottles of 1,000 NDC 42385-943-11
Carton with 64 (8 x 8) Unit-Dose Tablets NDC 42385-943-64
Carton with 100 (10 x 10) Unit-Dose Tablets NDC 42385-943-68
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.]
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myopathy and Rhabdomyolysis
Advise patients that atorvastatin may cause myopathy and rhabdomyolysis. Inform patients that the risk is also increased when taking certain types of medication or consuming large quantities of grapefruit juice and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Hepatic Dysfunction
Inform patients that atorvastatin may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see Warnings and Precautions (5.3)].
Increases in HbA1c and Fasting Serum Glucose Levels
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with atorvastatin. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see Warnings and Precautions (5.4)].
Pregnancy
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if atorvastatin should be discontinued [see Use in Specific Populations (8.1)].
Lactation
Advise patients that breastfeeding is not recommended during treatment with atorvastatin [see Use in Specific Populations (8.2)].
The brands listed are trademarks of their respective owners and are not trademarks of Laurus Labs Limited.
Dispense with Patient Information available at: https://www.laurusgenerics.us/images/Ator-patient-info.pdf
Manufactured for:
Laurus Generics Inc.
400 Connell Drive, Suite 5200
Berkeley Heights, NJ 07922
Manufactured by:
Laurus Labs Limited
Anakapalli-531011
India
PATIENT INFORMATION
| Patient Information
Atorvastatin Calcium (a tor'' va stat' in kal' see um) Tablets, USP, for oral use |
| What are atorvastatin calcium tablets?
Atorvastatin calcium tablets are a prescription medicine that contains a cholesterol lowering medicine (statin) called atorvastatin. Atorvastatin calcium tablets are used:
|
Do not take atorvastatin calcium tablets if you:
|
Before you take atorvastatin calcium tablets, tell your doctor about all of your medical conditions, including if you:
o ledipasvir plus sofosbuvir o simeprevir o saquinavir plus ritonavir o darunavir plus ritonavir o fosamprenavir o fosamprenavir plus ritonavir o elbasvir plus grazoprevir o letermovir o nelfinavir Ask your doctor or pharmacist for a list of medicines if you are not sure. Know all the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine. |
How should I take atorvastatin calcium tablets?
|
What should I avoid while taking atorvastatin calcium tablets?
|
| What are the possible side effects of atorvastatin calcium tablets?
Atorvastatin calcium tablets may cause serious side effects including:
These are not all the side effects of atorvastatin calcium tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
How do I store atorvastatin calcium tablets?
|
| General information about the safe and effective use of atorvastatin calcium tablets
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use atorvastatin calcium tablets for a condition for which it was not prescribed. Do not give atorvastatin calcium tablets to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information about atorvastatin calcium tablets, talk with your doctor. You can ask your pharmacist or doctor for information about atorvastatin calcium tablets that is written for health professionals. |
| What are the ingredients in atorvastatin calcium tablets?
Active Ingredient: atorvastatin calcium USP Inactive Ingredients: calcium carbonate, croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, Opadry White YS-1-7040 (hypromellose, polyethylene glycol, talc, titanium dioxide) and polysorbate 80. For more information, call Laurus Generics Inc. at 1-833-3-LAURUS (1-833-352-8787). This Patient package Information has been approved by the U.S. Food and Drug Administration. Dispense with Patient Information available at: https://www.laurusgenerics.us/images/Ator-patient-info.pdf Manufactured for: Laurus Generics Inc. 400 Connell Drive, Suite 5200 Berkeley Heights, NJ 07922 Manufactured by: Laurus Labs Limited Anakapalli-531011 India Revised: 01/2024 |
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 10 mg - Container Label (30's count)
NDC 42385-940-30
Atorvastatin Calcium Tablets, USP 10 mg*
30 Tablets Rx Only
Laurus Labs
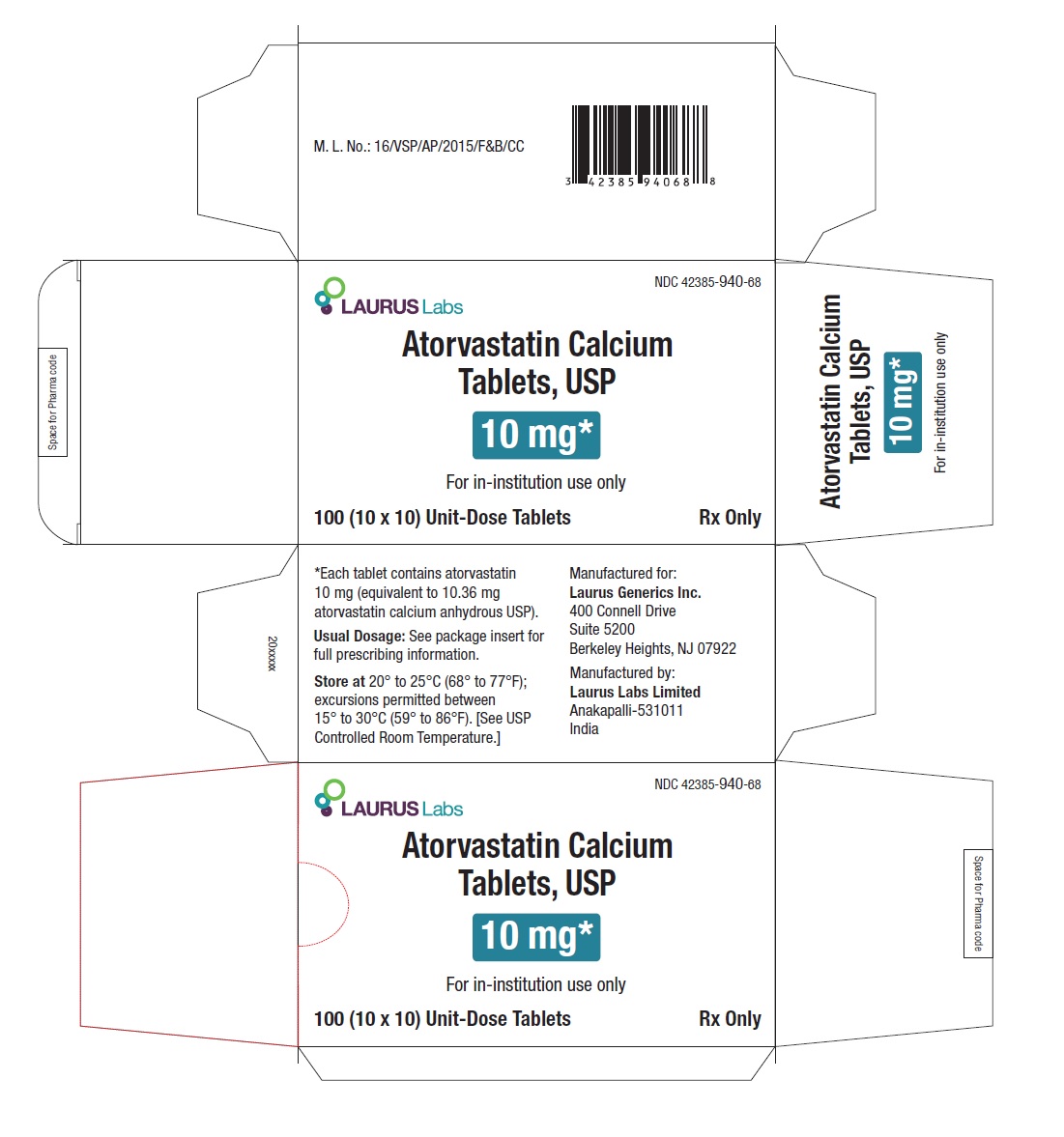
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -10 mg - Blister Carton - 100 (10 x 10) Unit-Dose Tablets
NDC 42385-940-68
Laurus Labs
Atorvastatin Calcium Tablets, USP 10 mg*
For in-institution use only
100 (10 x 10) Unit-Dose Tablets Rx Only
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 10 mg - Blister (1 x 10's count)
Rx Only NDC 42385-940-10
Atorvastatin Calcium Tablet, USP 10 mg
Mfd.by: Laurus Labs Limited
M.L.No.:16/VSP/AP/2015/F&B/CC

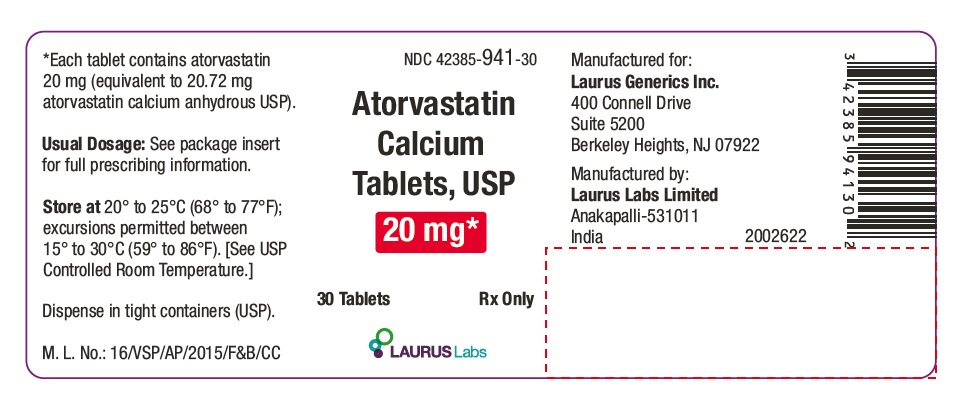
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 20 mg - Container Label (30's count)
NDC 42385-941-30
Atorvastatin Calcium Tablets, USP 20 mg*
30 Tablets Rx Only
Laurus Labs
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 20 mg - Blister Carton - 100 (10 x 10) Unit-Dose Tablets
NDC 42385-941-68
Laurus Labs
Atorvastatin Calcium Tablets, USP 20 mg*
For in-institution use only
100 (10 x 10) Unit-Dose Tablets Rx Only

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 20 mg - Blister (1 x 10's count)
Rx Only NDC 42385-941-10
Atorvastatin Calcium Tablet, USP 20 mg
Mfd.by: Laurus Labs Limited
M.L.No.:16/VSP/AP/2015/F&B/CC

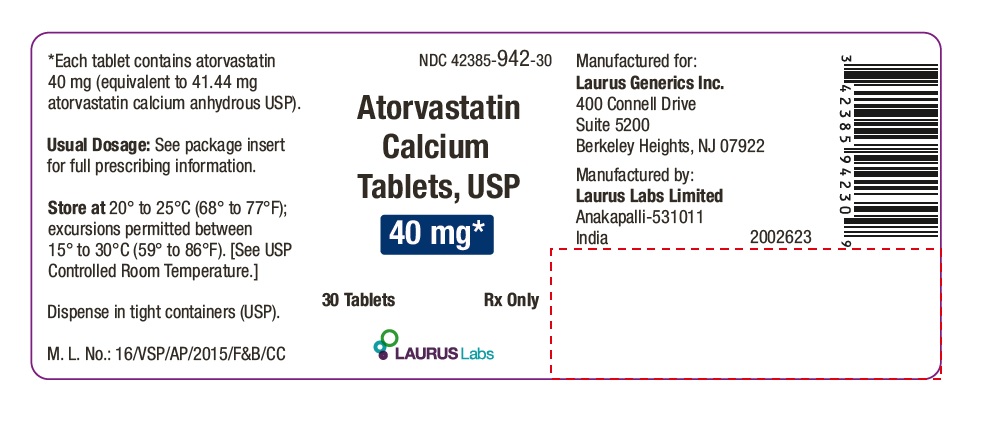
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 40 mg - Container Label (30's count)
NDC 42385-942-30
Atorvastatin Calcium Tablets, USP 40 mg*
30 Tablets Rx Only
Laurus Labs
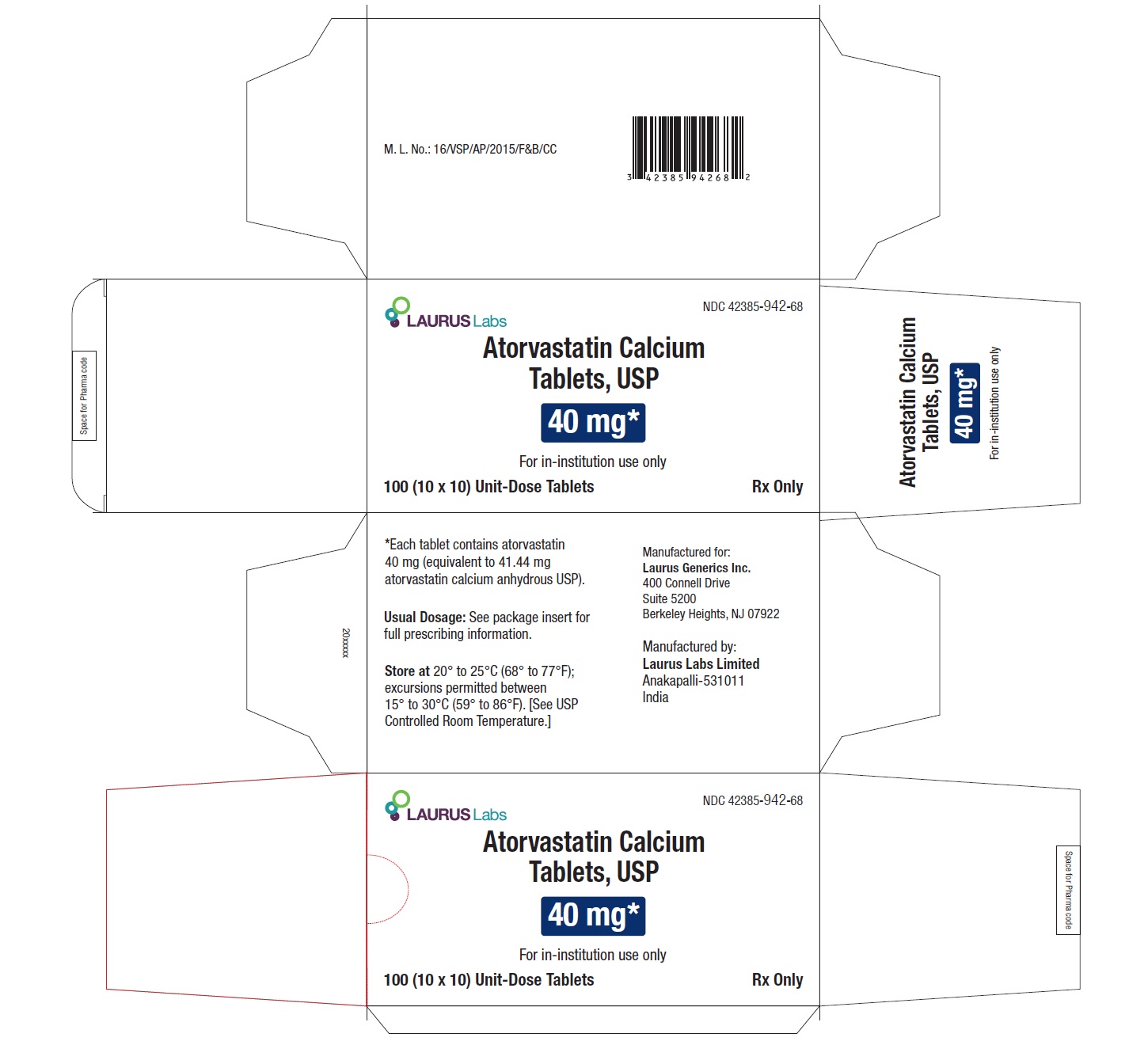
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 40 mg - Blister Carton - 100 (10 x 10) Unit-Dose Tablets
NDC 42385-942-68
Laurus Labs
Atorvastatin Calcium Tablets, USP 40 mg*
For in-institution use only
100 (10 x 10) Unit-Dose Tablets Rx Only
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 40 mg - Blister (1 x 10's count)
Rx Only NDC 42385-942-10
Atorvastatin Calcium Tablet, USP 40 mg
Mfd.by: Laurus Labs Limited
M.L.No.:16/VSP/AP/2015/F&B/CC
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Container Label (30's count)
NDC 42385-943-30
Atorvastatin Calcium Tablets, USP 80 mg*
30 Tablets Rx Only
Laurus Labs
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Blister Carton - 64 (8 x 8) Unit-Dose Tablets
NDC 42385-943-64
Laurus Labs
Atorvastatin Calcium Tablets, USP 80 mg*
For in-institution use only
64 (8 x 8) Unit-Dose Tablets Rx Only

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Blister (1 x 8's count)
Rx Only NDC 42385-943-08
Atorvastatin Calcium Tablet, USP 80 mg
Mfd.by: Laurus Labs Limited
M.L.No.:16/VSP/AP/2015/F&B/CC