DESCRIPTION: PNV-Select is a prescription prenatal dietary supplement tablet that contains multivitamins and minerals, which are delivered in the form of a white, oval, oil-and water-soluble, film-coated tablet debossed with “320” on one side.
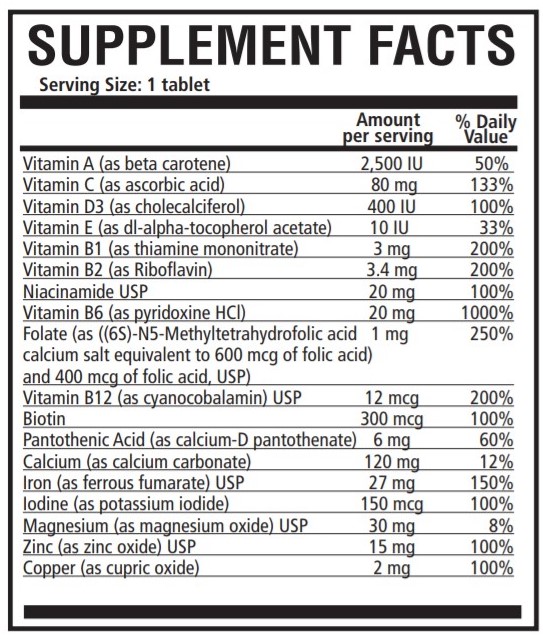
INDICATIONS: PNV-Select is a multivitamin/multimineral nutritional supplement indicated for use in the dietary management of patients with nutritional deficiencies or are in need of nutritional supplementation.
CONTRAINDICATIONS: This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
HOW SUPPLIED: PNV-Select is supplied in child-resistant bottles of 90 tablets (42192-320-90). The listed product is not a National Drug Code, but has merely been formatted to comply with standard industry practice for pharmacy and insurance computer systems.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature.]
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. NOTE: This is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the dietary ingredients, other ingredients and information provided herein.
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.
MANUFACTURED FOR:
Acella Pharmaceuticals, LLC
Alpharetta, GA 30005
1-800-541-4802
Rev 0719-03
