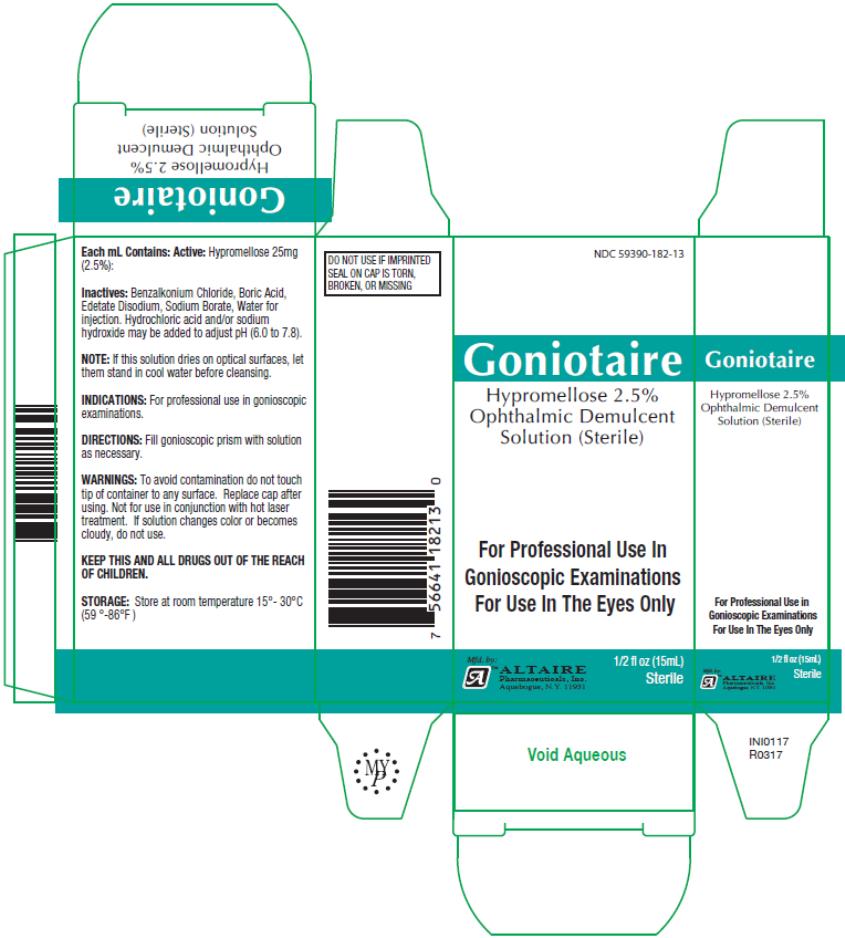
Altaire
Goniotaire
Hypromellose 2.5% Ophthalmic
Demulcent Solution
15mL
NDC 59390-182-13
Drug Facts
Each mL Contains:
Active:
Hypromellose 25mg (2.5%):
Inactives:
Benzalkonium Chloride, Boric Acid, Edetate Disodium, Sodium Borate, Water for injection. Hydrochloric Acid and/or sodium hydroxide may be added to adjust pH (6.0 to 7.8).
NOTE: If this solution dries on optical surfaces, let them stand in cool water before cleansing.
INDICATIONS:
For professional use in gonioscopic examinations.
DIRECTIONS:
Fill gonioscopic prism with solution as necessary.
WARNINGS:
To avoid contamination do not touch tip of container to any surface. Replace cap after using. Not for use in conjunction with hot laser treatment. If solution changes color or becomes cloudy, do not use.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
STORAGE:
Store at room temperature 15°- 30°C (59°- 86°F).
PRINCIPAL DISPLAY PANEL
NDC 59390-182-13
Goniotaire
Hypromellose 2.5%
Ophthalmic Demulcent
Solution (Sterile)
½ fl oz (15mL)
sterile
Altaire Pharmaceuticals Inc.
