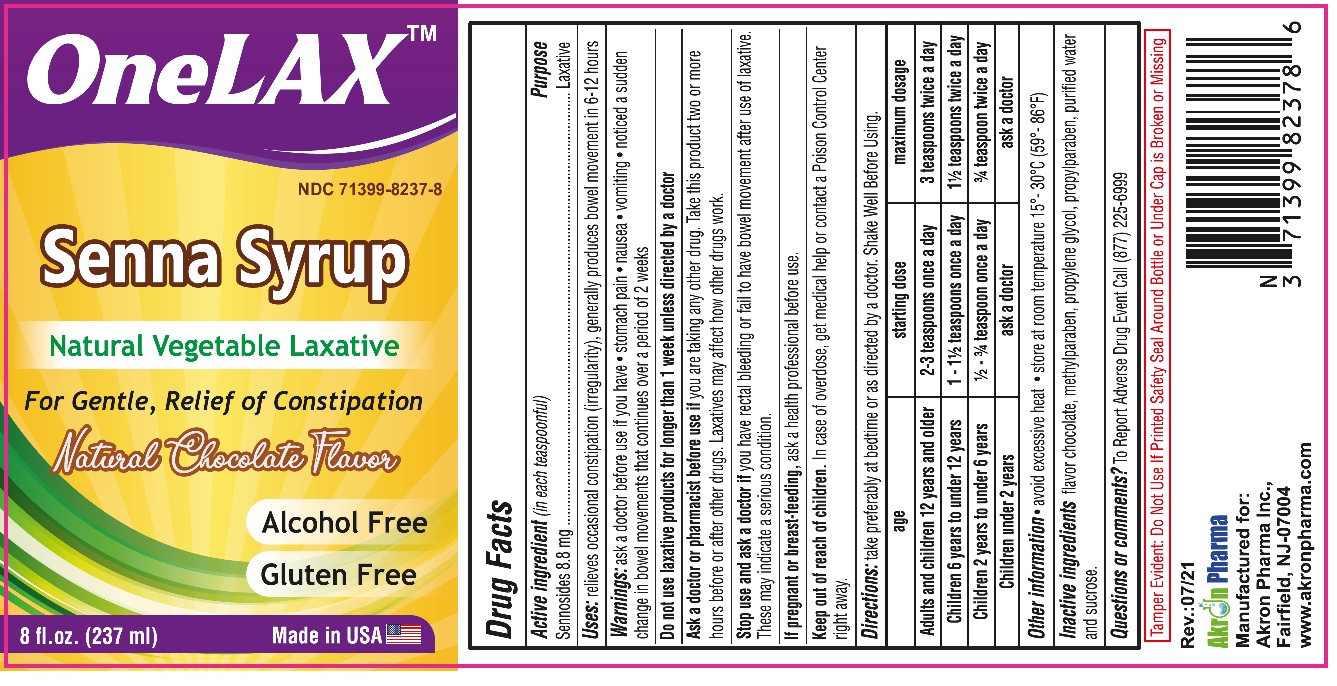
Active Ingredient
Active ingredient (in each 5 ml) purpose
Sennosides 8.8 mg …………………………................Laxative
Uses
relieves occasional constipation (irregularity)
generally causes bowel movement in 6 to 12 hours
| age | starting dose | maximum dosage |
| Mulls and children 12 yeart and older | 2-3 teaspoons once a day | 3 teaspoons twice a day |
| Children 6 years to under 12 yeart | 1 -11> teaspoons once a day | 11> teaspoons twice a day |
| Children 2 yeart to under 6 yeart | l> -¾teaspoon once a day | ¾ teaspoon twice a day |
| Children under 2 years | ask a doctor | ask a doctor |
Warnings
ask a doctor betore use rt you have • stomach pain • nausea • vomiting • noticed a sudden change in bowel movements that continues over a period of 2 weeks
Do not use laxative produe1s for longer than 1 week unless directed by a doctor