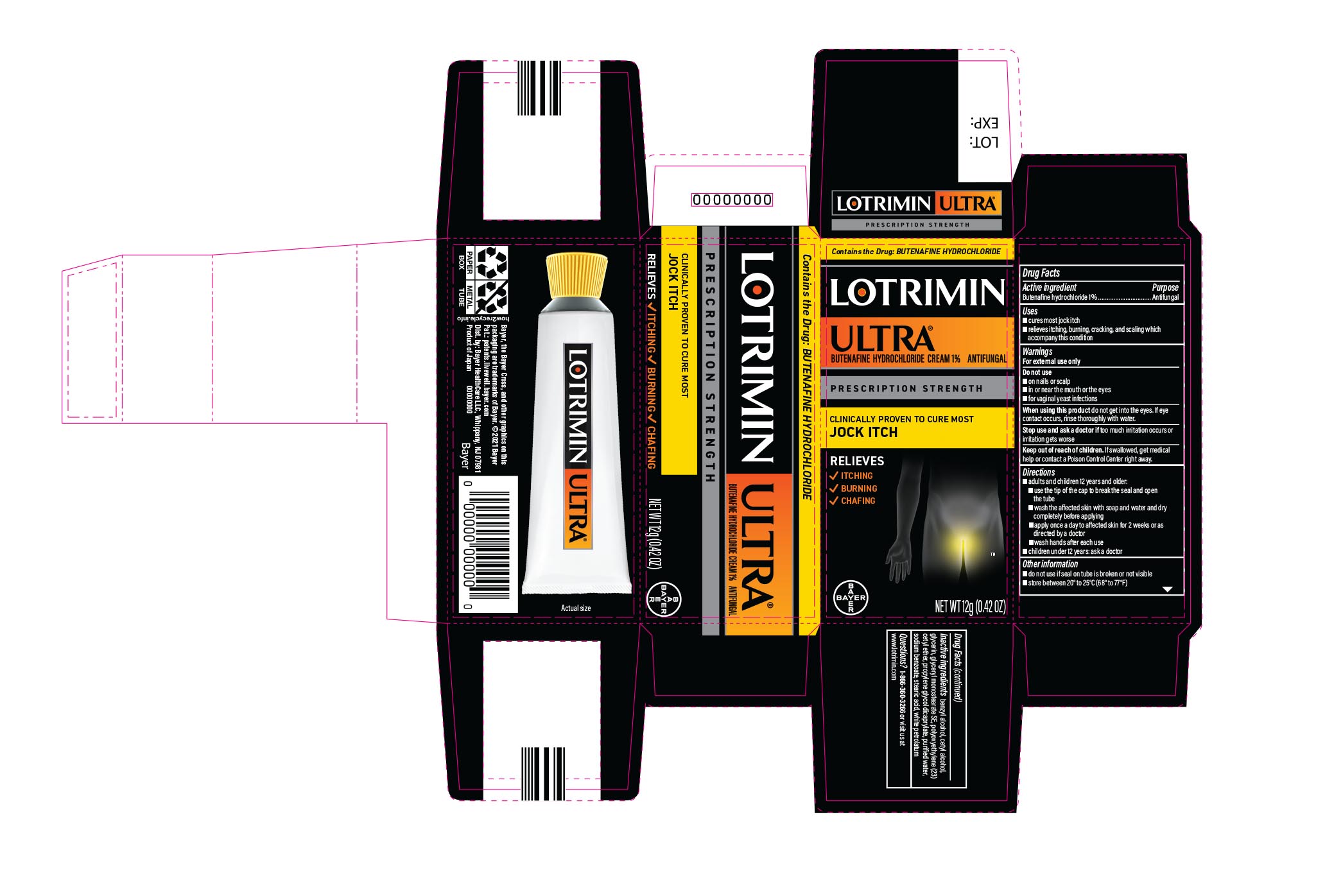
Active ingredient
Butenafine hydrochloride 1%
Uses
- cures most jock itch
- relieves itching, burning, cracking, and scaling which accompany this condition
Warnings
For external use only
Do not use
- on nails or scalp
- in or near the mouth or the eyes
- for vaginal yeast infections
When using this product do not get into the eyes. If eye contact occurs, rinse thoroughly with water.
Stop use and ask a doctor if too much irritation occurs or irritation gets worse
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over:
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
- apply once a day to affected skin for 2 weeks or as directed by a doctor
- wash hands after each use
- children under 12 years: ask a doctor
Other information
- do not use if seal on tube is broken or not visible
- store between 20° to 25°C (68° to 77°F)
Inactive ingredients
benzyl alcohol, cetyl alcohol, glycerin, glyceryl monostearate SE, polyoxyethylene (23) cetyl ether, propylene glycol dicaprylate, purified water, sodium benzoate, stearic acid, trolamine, white petrolatum
Distributed by Bayer HealthCare LLC, Whippany, NJ 07981
PRINCIPAL DISPLAY PANEL - 12 g Tube Carton
LOTRIMIN ULTRA®
butenafine hydrochloride cream 1% ANTIFUNGAL NET WT 12g (0.42 OZ)
Bayer Healthcare LLC.
