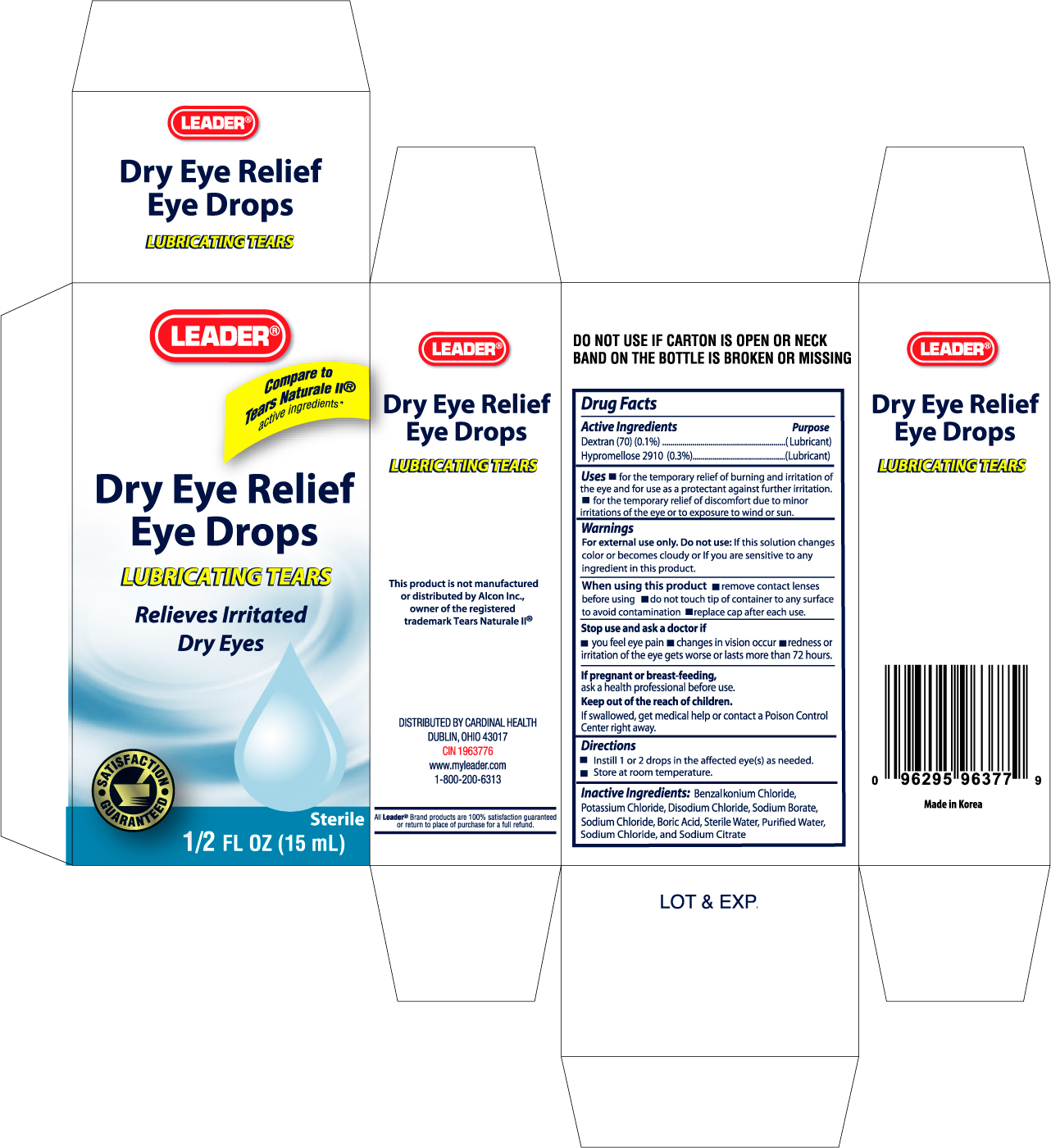
Active Ingredients Purpose
Dextran 70 (0.1%).........................................................................(Lubricant)
Hypromellose 2910 (0.3%).............................................................(Lubricant)
Uses
- for the temporary relief of burning and irritation of the eye and for use as a protectant against further irritation.
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun.
Warnings
For external use only. Do not use: If this solution changes color or becomes cloudy or if you are sensitive to any ingredient in this product.
When using this product
- remove contact lenses before using
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel pain
- changes in vision occur
- redness or irritation of the eye gets worse or lasts more than 72 hours
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
 Enter section text here
Enter section text here