Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive ingredients
sorbitol, purified water, hydrated silica, glycerin, xylitol, charcoal powder, flavor, xanthan gum, titanium dioxide, cocos nucifera (coconut) oil1, cocamidopropyl betaine, sodium cocoyl glutamate and stevia rebaudiana leaf extract1
- 1
- certified organic
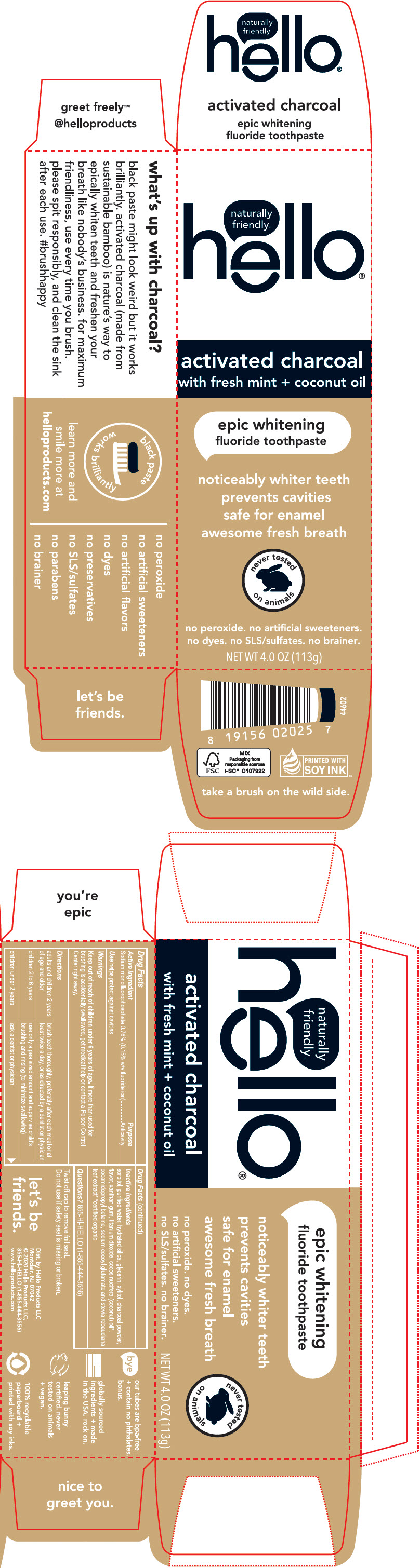
PRINCIPAL DISPLAY PANEL - 113 g Tube Carton
naturally
friendly™
hello®
activated charcoal
with fresh mint + coconut oil
epic whitening
fluoride toothpaste
noticeably whiter teeth
prevents cavities
safe for enamel
awesome fresh breath
never tested
on animals
no peroxide. no artificial sweeteners.
no dyes. no SLS/sulfates. no brainer.
NET WT 4.0 OZ (113g)