DESCRIPTION
FML-S® ophthalmic suspension is a topical anti-inflammatory/anti-infective product for ophthalmic use.
Chemical Names:
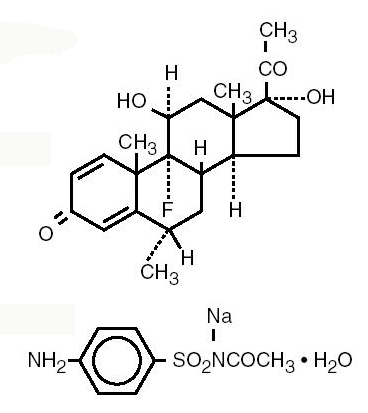
Fluorometholone:
9-Fluoro-11β,17-dihydroxy-6α–methylpregna-1,4-diene-3,20-dione.
Sulfacetamide sodium:
N-Sulfanilyacetamide monosodium salt monohydrate.
Structural Formulas:
Contains: Actives: fluorometholone 0.1%; and sulfacetamide sodium 10%. Preservative: benzalkonium chloride 0.006%. Inactives: edetate disodium; polysorbate 80; polyvinyl alcohol (1.4%); povidone; purified water; sodium chloride; sodium phosphate dibasic; sodium phosphate, monobasic; sodium thiosulfate; and hydrochloric acid and/or sodium hydroxide to adjust the pH to 7.0 to 7.5.
CLINICAL PHARMACOLOGY
Corticosteroids suppress the inflammatory response to a variety of agents and they probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. Since corticosteroids may inhibit the body's defense mechanism against infection, a concomitant antimicrobial drug may be used when this inhibition is considered to be clinically significant in a particular case.
Corticosteroids are capable of producing a rise in intraocular pressure. In clinical studies of documented steroid-responders, fluorometholone demonstrated a significantly longer average time to produce a rise in intraocular pressure than dexamethasone phosphate; however, in a small percentage of individuals, a significant rise in intraocular pressure occurred within one week. The ultimate magnitude of the rise was equivalent for both drugs.
The anti-infective component in FML-S® ophthalmic suspension is included to provide action against specific organisms susceptible to it. Sulfacetamide sodium is active in vitro against susceptible strains of the following microorganisms: Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae, Klebsiella species, and Enterobacter species. Some strains of these bacteria may be resistant to sulfacetamide or resistant strains may emerge in vivo.
When a decision to administer both a corticosteroid and an antimicrobial is made, the administration of such drugs in combination has the advantage of greater patient compliance and convenience, with the added assurance that the appropriate dosage of both drugs is administered. When both types of drugs are in the same formulation, compatibility of ingredients is assured and the correct volume of drug is delivered and retained.
The relative potency of corticosteroid formulations depends on the molecular structure, concentration, and release from the vehicle.
INDICATIONS AND USAGE
FML-S® ophthalmic suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe, where the inherent risk of steroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns or penetration of foreign bodies.
The use of a combination drug with an anti-infective component is indicated where the risk of superficial ocular infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
The anti-infective drug in this product, sulfacetamide, is active against the following common bacterial eye pathogens: Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae, Klebsiella species, and Enterobacter species.
This product does not provide adequate coverage against Neisseria species and Serratia marcescens. A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
CONTRAINDICATIONS
FML-S® ophthalmic suspension is contraindicated in most viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. FML-S® ophthalmic suspension is also contraindicated in individuals with known or suspected hypersensitivity to any of the ingredients of this preparation, to sulfonamides and to other corticosteroids.
WARNINGS
Prolonged use of corticosteroids may result in glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and in posterior subcapsular cataract formation. Prolonged use may also suppress the host immune response and thus increase the hazard of secondary ocular infections.
Various ocular diseases and long-term use of topical corticosteroids have been known to cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation.
Acute purulent infections of the eye may be masked or activity enhanced by the presence of corticosteroid medication.
If this product is used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in children and uncooperative patients. Steroids should be used with caution in the presence of glaucoma. Intraocular pressure should be checked frequently.
The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation.
Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution; frequent slit lamp microscopy is recommended.
FML-S® ophthalmic suspension is not for injection. It should never be injected subconjunctivally, nor should it be directly introduced into the anterior chamber of the eye.
FATALITIES HAVE OCCURRED, ALTHOUGH RARELY, DUE TO SEVERE REACTIONS TO SULFONAMIDES INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS. Sensitizations may recur when a sulfonamide is readministered, irrespective of the route of administration. If signs of hypersensitivity or other serious reactions occur, discontinue use of this preparation (see ADVERSE REACTIONS).
Cross-sensitivity among corticosteroids has been demonstrated.
A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
PRECAUTIONS
General:
The initial prescription and renewal of the medication order beyond 20 milliliters of FML-S® ophthalmic suspension should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy and, where appropriate, fluorescein staining. If signs and symptoms fail to improve after two days, the patient should be re-evaluated.
As fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid applications, fungal invasion should be suspected in any persistent corneal ulceration where a corticosteroid has been used or is in use. Fungal cultures should be taken when appropriate.
If this product is used for 10 days or longer, intraocular pressure should be monitored (see WARNINGS).
Prolonged use of topical anti-bacterial agents may give rise to overgrowth of nonsusceptible organisms including fungi. Bacterial resistance to sulfonamides may also develop.
The effectiveness of sulfonamides may be reduced by the para-aminobenzoic acid present in purulent exudates.
Information for Patients:
If inflammation or pain persists longer than 48 hours or becomes aggravated, the patient should be advised to discontinue use of the medication and consult a physician.
This product is sterile when packaged. To prevent contamination, care should be taken to avoid touching the bottle tip to eyelids or to any other surface. The use of this bottle by more than one person may spread infection. Keep bottle tightly closed when not in use. Keep out of the reach of children.
Carcinogenesis, mutagenesis, impairment of fertility:
No studies have been conducted in animals or in humans to evaluate the possibility of these effects with fluorometholone or sulfacetamide.
Pregnancy:
Teratogenic effects: Pregnancy Category C:
Animal studies have not been conducted with FML-S® ophthalmic suspension. Fluorometholone has been shown to be embryocidal and teratogenic in rabbits when administered at low multiples of the human dose. Fluorometholone was applied ocularly to rabbits daily on days 6-18 of gestation and dose-related fetal loss and fetal abnormalities including cleft palate, deformed rib cage, anomalous limbs and neural abnormalities such as encephalocele, craniorachischisis, and spina bifida were observed. Kernicterus may be precipitated in infants by sulfonamides being given systemically during the third trimester of pregnancy. There are no adequate and well-controlled studies of FML-S® ophthalmic suspension in pregnant women, and it is not known whether FML-S® ophthalmic suspension can cause fetal harm when administered to a pregnant woman. FML-S® ophthalmic suspension should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers:
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids appear in breast milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Systemically administered sulfonamides are capable of producing kernicterus in infants of lactating women. Because of the potential for serious adverse reactions in nursing infants from FML-S® ophthalmic suspension, a decision should be made whether to discontinue nursing or to discontinue the medication.
ADVERSE REACTIONS
Adverse reactions have occurred with corticosteroid/anti-infective combination drugs which can be attributed to the corticosteroid component, the anti-infective component, or the combination. Exact incidence figures are not available, since no denominator of treated patients is available.
Reactions occurring most often from the presence of the anti-infective ingredient are allergic sensitizations. Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias (See WARNINGS). Sulfacetamide sodium may cause local irritation.
The reactions due to the corticosteroid component in decreasing order of frequency are: elevation of intraocular pressure (IOP) with possible development of glaucoma, and infrequent optic nerve damage; posterior subcapsular cataract formation; and delayed wound healing.
Corticosteroid-containing preparations have also been reported to cause perforation of the globe. Keratitis, conjunctivitis, corneal ulcers, and conjunctival hyperemia have occasionally been reported following local use of steroids.
Secondary Infection: The development of secondary infection has occurred after use of combinations containing corticosteroids and antimicrobials. Fungal infections of the cornea are particularly prone to develop coincidentally with long-term applications of corticosteroids. When signs of chronic ocular inflammation persist following prolonged corticosteroid dosing, the possibility of fungal infections of the cornea should be considered.
Secondary bacterial ocular infection following suppression of host responses also occurs.
DOSAGE AND ADMINISTRATION
One drop of FML-S® ophthalmic suspension should be instilled into the conjunctival sac four times daily. Care should be taken not to discontinue therapy prematurely. If signs and symptoms fail to improve after two days, the patient should be re-evaluated (see PRECAUTIONS).
The dosing of FML-S® ophthalmic suspension may be reduced, but care should be taken not to discontinue therapy prematurely. In chronic conditions, withdrawal of treatment should be carried out by gradually decreasing the frequency of applications.
HOW SUPPLIED
FML-S® (fluorometholone and sulfacetamide sodium ophthalmic suspension, USP) 0.1%/10% is supplied sterile in opaque white LDPE plastic bottles with droppers with white high impact polystyrene (HIPS) caps as follows:
- 5 mL in 10 mL bottle - NDC 11980-422-05
- 10 mL in 15 mL bottle - NDC 11980-422-10
Note: Store at 15°C - 30°C (59° -86°F). Protect from freezing and light. SHAKE WELL BEFORE USING. Do not use suspension if it is dark brown.
Rx Only
Rev February 2005
© 2005 Allergan, Inc.
® marks owned by Allergan, Inc.
7767X
71745US10P