SPL UNCLASSIFIED
BUFFERED
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Penicillin G Potassium and other antibacterial drugs, Penicillin G Potassium should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Buffered Penicillin G Potassium for Injection, USP is sterile penicillin G potassium powder for reconstitution. It is an antibacterial agent intended for intravenous or intramuscularly use.
Chemically, Penicillin G Potassium is monopotassium (2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo (3.2.0) heptane-2-carboxylate, and has the following chemical structure:
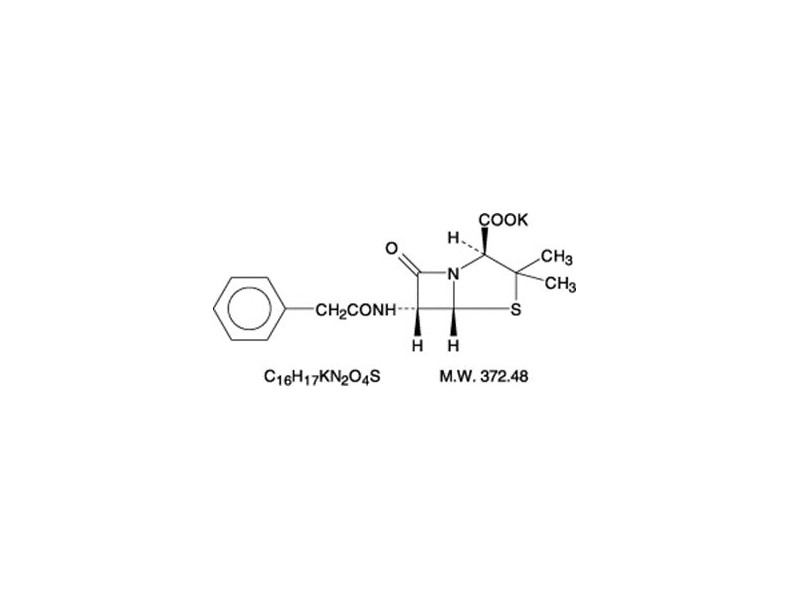
Penicillin G potassium, a water soluble benzylpenicillin, is a white to almost white crystalline powder which is almost odorless and/or after reconstitution a colorless solution. The pH of freshly constituted solutions usually ranges from 6 to 8.5. Sodium citrate and citric acid have been added as a buffer.
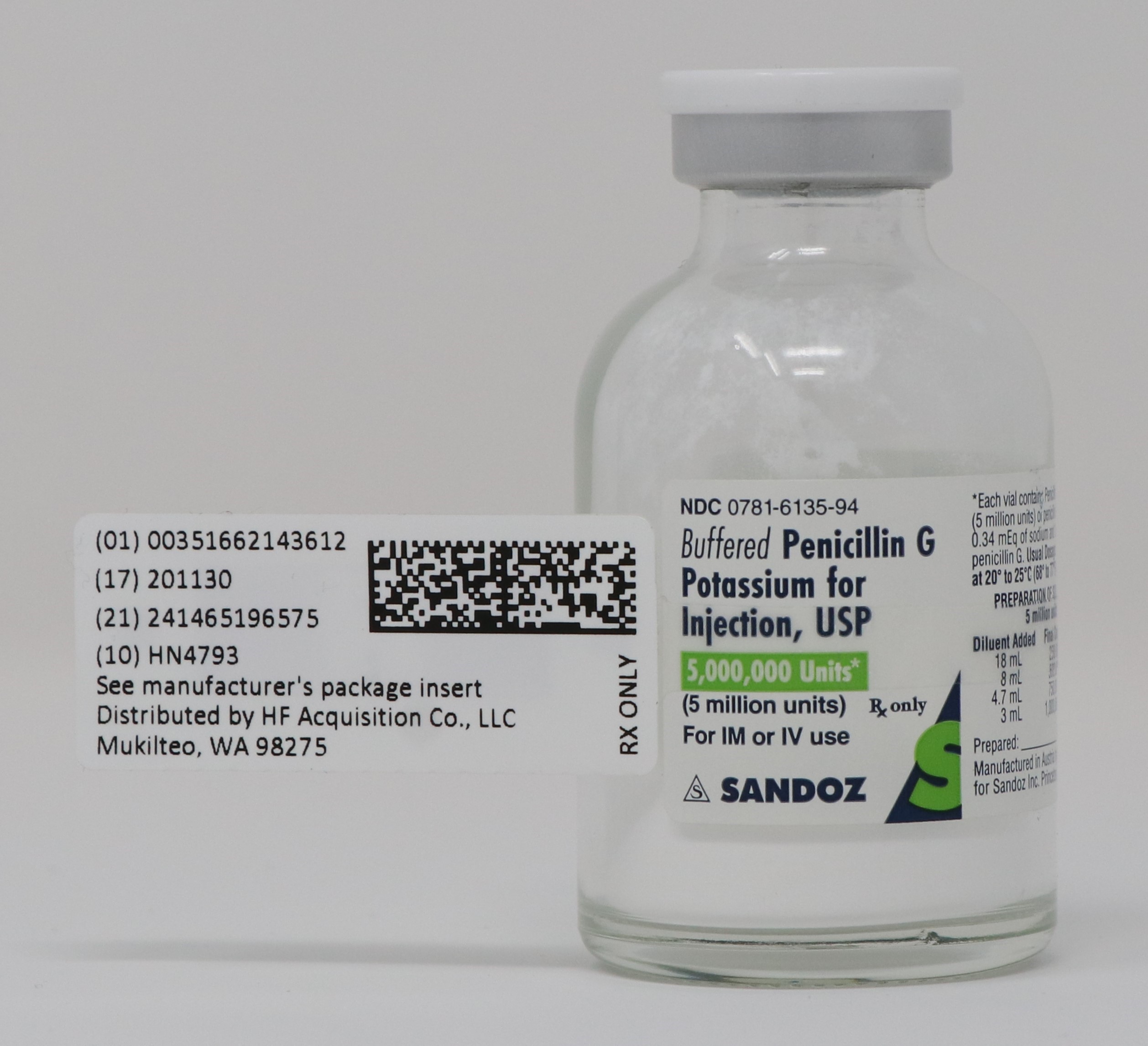
Buffered Penicillin G Potassium for Injection, USP is supplied in vials equivalent to 1,000,000 units (1 million units), 5,000,000 units (5 million units), or 20,000,000 units (20 million units) of penicillin G as the potassium salt. Each million unit contains approximately 7.9 milligrams of sodium (0.34 mEq) and 65.6 milligrams of potassium (1.68 mEq).
CLINICAL PHARMACOLOGY
After an intravenous infusion of penicillin G, peak serum concentrations are attained immediately after completion of the infusion. In a study of ten patients administered a single 5 million unit dose of penicillin G intravenously over 3 to 5 minutes, the mean serum concentrations were 400 mcg/mL, 273 mcg/mL and 3 mcg/mL at 5 to 6 minutes, 10 minutes and 4 hours after completion of the injection, respectively. In a separate study, five healthy adults were administered one million units of penicillin G intravenously, either as a bolus over 4 minutes or as an infusion over 60 minutes. The mean serum concentration eight minutes after completion of the bolus was 45 mcg/mL and eight minutes after completion of the infusion was 14.4 mcg/mL.
The mean β-phase serum half-life of penicillin G administered by the intravenous route in ten patients with normal renal function was 42 minutes, with a range of 31 to 50 minutes.
The clearance of penicillin G in normal individuals is predominantly via the kidney. The renal clearance, which is extremely rapid, is the result of glomerular filtration and active tubular transport, with the latter route predominating. Urinary recovery is reported to be 58 to 85% of the administered dose. Renal clearance of penicillin is delayed in premature infants, neonates and in the elderly due to decreased renal function. The serum half-life of penicillin G correlates inversely with age and clearance of creatinine and ranges from 3.2 hours in infants 0 to 6 days of age to 1.4 hours in infants 14 days of age or older.
Nonrenal clearance includes hepatic metabolism and, to a lesser extent, biliary excretion. The latter routes become more important with renal impairment.
Probenecid blocks the renal tubular secretion of penicillin. Therefore, the concurrent administration of probenecid prolongs the elimination of penicillin G and, consequently, increases the serum concentrations.
Penicillin G is distributed to most areas of the body including lung, liver, kidney, muscle, bone and placenta. In the presence of inflammation, levels of penicillin in abscesses, middle ear, pleural, peritoneal and synovial fluids are sufficient to inhibit most susceptible bacteria. Penetration into the eye, brain, cerebrospinal fluid (CSF) or prostate is poor in the absence of inflammation. With inflamed meninges, the penetration of penicillin G into the CSF improves, such that the CSF/serum ratio is 2 to 6%. Inflammation also enhances its penetration into the pericardial fluid. Penicillin G is actively secreted into the bile resulting in levels at least 10 times those achieved simultaneously in serum. Penicillin G penetrates poorly into human polymorphonuclear leukocytes.
In the presence of impaired renal function, the β-phase serum half-life of penicillin G is prolonged. β-phase serum half-lives of one to two hours were observed in azotemic patients with serum creatinine concentrations <3 mg/100 mL and ranged as high as 20 hours in anuric patients. A linear relationship, including the lowest range of renal function, is found between the serum elimination rate constant and renal function as measured by creatinine clearance.
In patients with altered renal function, the presence of hepatic insufficiency further alters the elimination of penicillin G. In one study, the serum half-lives in two anuric patients (excreting <400 mL urine/day) were 7.2 and 10.1 hours. A totally anuric patient with terminal hepatic cirrhosis had a penicillin half-life of 30.5 hours, while another patient with anuria and liver disease had a serum half-life of 16.4 hours. The dosage of penicillin G should be reduced in patients with severe renal impairment, with additional modifications when hepatic disease accompanies the renal impairment.
Hemodialysis has been shown to reduce penicillin G serum levels.
Microbiology
Penicillin G is bactericidal against penicillin-susceptible microorganisms during the stage of active multiplication. It acts by inhibiting biosynthesis of cell-wall mucopeptide. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci. Penicillin G is highly active in vitro against staphylococci (except penicillinase-producing strains), streptococci (groups A, B, C, G, H, L and M), pneumococci and Nelsseria meningitidis. Other organisms susceptible in vitro to penicillin G are Nelsseria gonorrhoeae, Corynebacterium diphtheriae, Bacillus anthracis, clostridia, Actinomyces species, Spirillum minus, Streptobacillus monillformis, Listeria monocytogenes, and leptospira; Treponema pallidum is extremely susceptible.
Some species of gram-negative bacilli were previously considered susceptible to very high intravenous doses of penicillin G (up to 80 million units/day) including some strains of Escherichia coli, Proteus mirabilis, salmonella, shigella, Enterobacter aerogenes (formerly Aerobacter aerogenes) and Alcaligenes faecalis. Penicillin G is no longer considered a drug of choice for infections caused by these organisms.
Susceptibility Testing
Diffusion techniques
The use of antibiotic disk susceptibility test methods which measure zone diameter give an accurate estimation of antibiotic susceptibility. One such standard procedure1 which has been recommended for use with disks to test susceptibility of organisms to penicillin G uses the 10 Unit (U) penicillin disk. Interpretation involves the correlation of the diameters obtained in the disk test with the minimum inhibitory concentration (MIC) for penicillin G.
Reports from the laboratory giving results of the standard single-disk susceptibility test with a 10 U penicillin disk should be interpreted according to the following criteria:
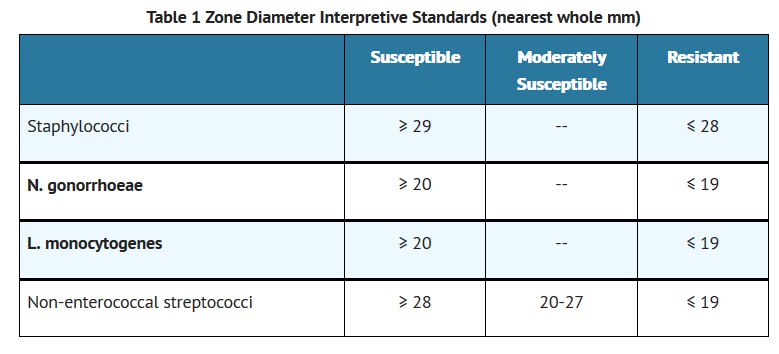
A report of “susceptible” indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of “moderately susceptible” suggests that the organism would be susceptible if high dosage is used or if the infection is confined to tissue and fluids (e.g., urine) in which high antibiotic levels are obtained. A report of “resistant” indicates that achievable concentrations are unlikely to be inhibitory and other therapy should be selected.
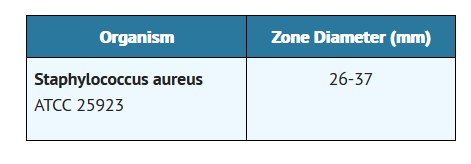
Standardized procedures require the use of laboratory control organisms. The 10 U penicillin G disk should give the following zone diameters:
Dilution techniques
When using a standardized dilution method2 (broth, agar, microdilution) or equivalent an organism may be considered susceptible if the minimum inhibitory concentration (MIC) values are interpreted according to the following table:
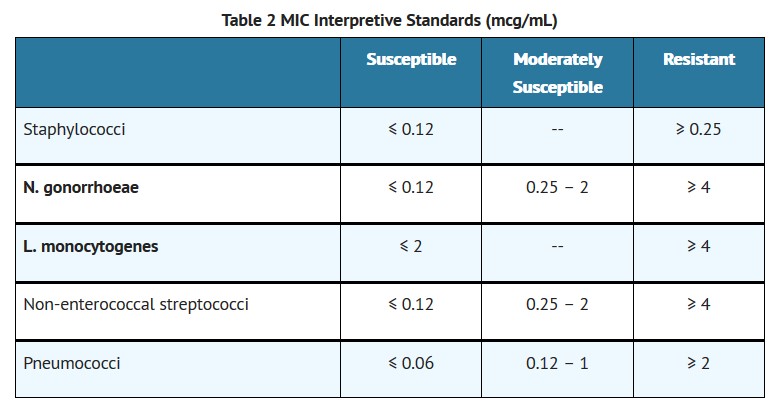
MIC test results should be interpreted according to the concentration of penicillin G that can be attained in blood (serum), tissue, and body fluids.
As with standard diffusion techniques, dilution methods require the use of laboratory control organisms. Standard penicillin G powder should provide the following MIC values:
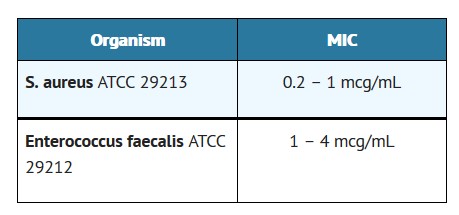
For anaerobic bacteria the MIC of penicillin G can be determined by agar or broth dilution (including microdilution) techniques3.
INDICATIONS & USAGE
Therapy
Buffered Penicillin G Potassium for Injection, USP is indicated in the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below. Appropriate culture and susceptibility tests should be done before treatment in order to isolate and identify organisms causing infection and to determine their susceptibility to penicillin G. Therapy with Buffered Penicillin G Potassium for Injection, USP may be initiated before results of such tests are known when there is reason to believe the infection may involve any of the organisms listed below, however, once these results become available, appropriate therapy should be continued.
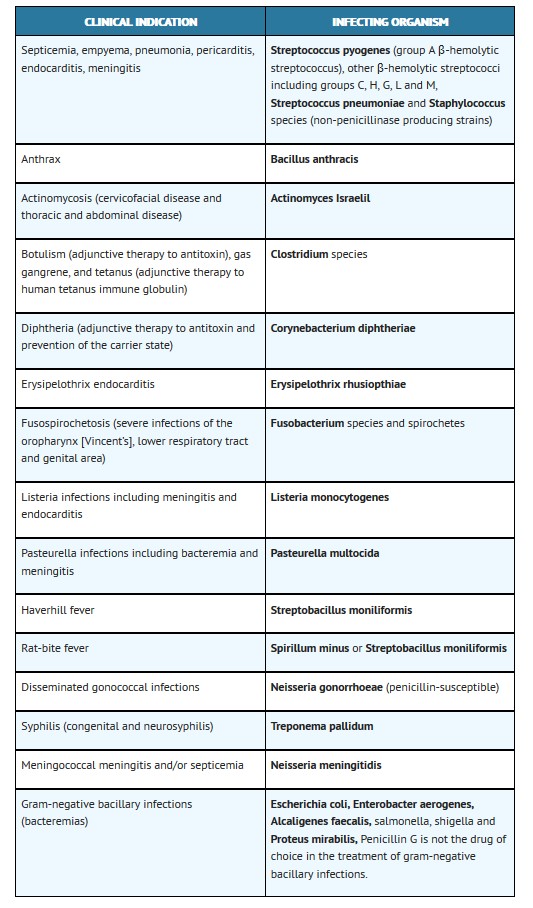
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Penicillin G Potassium and other antibacterial drugs, Penicillin G Potassium should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
A history of hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH PENICILLIN G, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, PENICILLIN G SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including penicillin G, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation and treatment with an antibacterial drug effective against C. difficile.
PRECAUTIONS
General
Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma (see WARNINGS). Whenever allergic reactions occur, penicillin should be withdrawn unless, in the opinion of the physician, the condition being treated is lifethreatening and amenable only to penicillin therapy.
Buffered Penicillin G Potassium for Injection, USP by the intravenous route in high doses (above 10 million units) should be administered slowly because of the potential adverse effects of electrolyte imbalance from the potassium content of the penicillin. Buffered Penicillin G Potassium for Injection, USP contains approximately 6.8 milligrams of sodium (0.3 mEq) and 65.6 milligrams of potassium (1.68 mEq) per million units of penicillin G.
The use of antibiotics may promote overgrowth of nonsusceptible organisms, including fungi. Indwelling intravenous catheters encourage superinfections. Should superinfection occur, appropriate measures should be taken.
When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy.
Prescribing Penicillin G Potassium in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Laboratory Tests
Periodic assessment of organ system function, including frequent evaluation of electrolyte balance, hepatic, renal and hematopoietic systems, and cardiac and vascular status should be performed during prolonged therapy with high doses of intravenous penicillin G (see ADVERSE REACTIONS). If any impairment of function is suspected or known to exist, a reduction in the total dosage should be considered (see DOSAGE AND ADMINISTRATION).
In suspected staphylococcal infections, proper laboratory studies, including susceptibility tests should be performed.
All infections due to Group A beta-hemolytic streptococci should be treated for at least 10 days.
Patients being treated for gonococcal infection should have a serologic test for syphilis before receiving penicillin. All cases of penicillin treated syphilis should receive adequate follow-up including clinical and serological examinations. The recommended follow-up varies with the stage of syphilis being treated. See CDC recommendations.4
Drug Interactions
Bacteriostatic antibacterials (i.e., chloramphenicol, erythromycins, sulfonamides or tetracyclines) may antagonize the bactericidal effect of penicillin, and concurrent use of these drugs should be avoided. This has been documented in vitro, however, the clinical significance of this interaction is not well-documented.
Penicillin blood levels may be prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins.
Other drugs may compete with Penicillin G for renal tubular secretion and thus prolong the serum half-life of penicillin. These drugs include: aspirin, phenylbutazone, sulfonamides, indomethacin, thiazide diuretics, furosemide and ethacrynic acid.
Drug/Laboratory Test Interactions
After treatment with penicillin G, a false-positive reaction for glucose in the urine may occur with Benedict’s solution, Fehling’s solution or Clinitest® tablet, but not with the enzyme-based tests, such as Clinistix®.
Penicillin G has been associated with pseudoproteinuria by certain test methods.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been conducted with this drug.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to penicillin G. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate and well controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Penicillins are excreted in human milk. Caution should be exercised when penicillins are administered to a nursing woman.
Pediatric Use
Incompletely developed renal function in newborns may delay elimination of penicillin; therefore, appropriate reductions in the dosage and frequency of administration should be made in these patients. All newborns treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects (see PRECAUTIONS).
Pediatric doses are generally determined on a weight basis and should be calculated for each patient individually. Recommended guidelines for pediatric dosages are presented in DOSAGE AND ADMINISTRATION.
The potential for toxic effects in children from chemicals that may leach from the single dose premixed intravenous preparation in plastic containers has not been evaluated.
Patients should be counseled that antibacterial drugs including Penicillin G Potassium should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Penicillin G Potassium is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Penicillin G Potassium or other antibacterial drugs in the future.
ADVERSE REACTIONS
Body as a whole
The Jarisch-Herxheimer reaction is a systemic reaction, that may occur after the initiation of penicillin therapy in patients with syphilis or other spirochetal infections (i.e., Lyme disease and Relapsing fever). The reaction begins one to two hours after initiation of therapy and disappears within 12 to 24 hours. It is characterized by fever, chills, myalgias, headache, exacerbation of cutaneous lesions, tachycardia, hyperventiliation, vasodilation with flushing and mild hypotension. The pathogenesis of the Herxheimer reaction may be due to the release from the spirochaete of host stable pyrogen.
Hypersensitivity reactions
The reported incidence of allergic reactions to all penicillins ranges from 0.7 to 10 percent in different studies (see WARNINGS). Sensitization is usually the result of previous treatment with a penicillin, but some individuals have had immediate reactions when first treated. In such cases, it is postulated that prior exposure to penicillin may have occurred via trace amounts present in milk or vaccines.
Two types of allergic reactions to penicillin are noted clinically – Immediate and delayed.
Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angloneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse and death (see WARNINGS). Such immediate anaphylactic reactions are very rare and usually occur after parenteral therapy, but a few cases of anaphylaxis have been reported following oral therapy. Another type of immediate reaction, an accelerated reaction, may occur between 20 minutes and 48 hours after administration and may include urticaria, pruritus, fever and, occasionally, laryngeal edema.
Delayed reactions to penicillin therapy usually occur within 1 to 2 weeks after initiation of therapy. Manifestations include serum sickness-like symptoms, i.e., fever, malaise, urticaria, myalgia, arthralgia, abdominal pain and various skin rashes, ranging from maculopapular eruptions to exfoliative dermatitis.
Contact dermatitis has been observed in individuals who prepare penicillin solutions.
Gastrointestinal system
Pseudomembranous colitis has been reported with the onset occurring during or after penicillin G treatment. Nausea, vomiting, stomatitis, black or hairy tongue, and other symptoms of gastrointestinal irritation may occur, especially during oral therapy.
Hematologic system
Reactions include neutropenia, which resolves after penicillin therapy is discontinued; Coombspositive hemolytic anemia, an uncommon reaction, occurs in patients treated with intravenous penicillin G in doses greater than 10 million units/day and who have previously received large doses of the drug; and with large doses of penicillin, a bleeding diathesis, can occur secondary to platelet dysfunction.
Metabolic
Buffered Penicillin G Potassium for Injection, USP (1 million units contains 0.3 mEq of sodium and 1.68 mEq of potassium) may cause serious and even fatal electrolyte disturbances, i.e., hyperkalemia, when given intravenously in large doses.
Nervous system
Neurotoxic reactions including hyperreflexia, myoclonic twitches, seizures and coma have been reported following the administration of massive intravenous doses, and are more likely in patients with impaired renal function.
Urogenital system
Renal tubular damage and interstitial nephritis have been associated with large intravenous doses of penicillin G. Manifestations of this reaction may include fever, rash, eosinophilia, proteinuria, eosinophiluria, hematuria and a rise in serum urea nitrogen. Discontinuation of penicillin G results in resolution in the majority of patients.
Local reactions
Phlebitis and thrombophlebitis may occur with intravenous administration.
OVERDOSAGE
Dose related toxicity may arise with the use of massive doses of intravenous penicillins (40 to 100 million units per day), particularly in patients with severe renal impairment (see PRECAUTIONS). The manifestations may include agitation, confusion, asterixis, hallucinations, stupor, coma, multifocal myoclonus, seizures and encephalopathy. Hyperkalemia is also possible (see ADVERSE REACTIONS – Metabolic).
In case of overdosage, discontinue penicillin, treat symptomatically and institute supportive measures as required. If necessary, hemodialysis may be used to reduce blood levels of penicillin G, although the degree of effectiveness of this procedure is questionable.
DOSAGE & ADMINISTRTION
Buffered Penicillin G Potassium for Injection, USP may be given intravenously or intramuscularly. The usual dose recommendations are as follows:
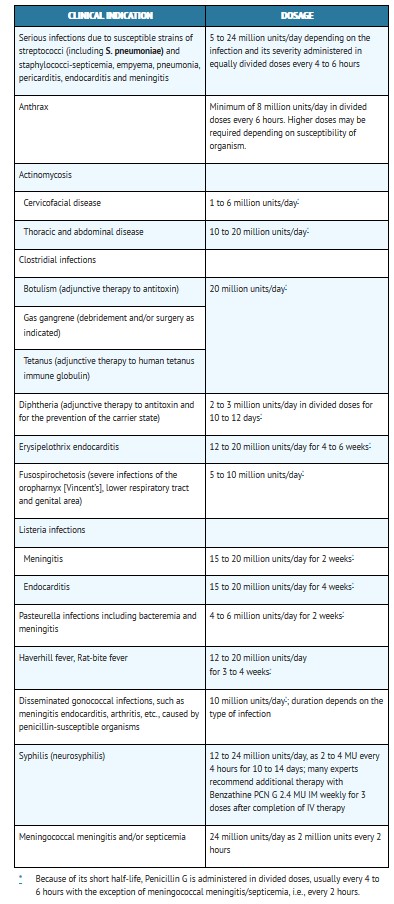
Pediatric patients
This product should not be administered to patients requiring less than one million units per dose. (see PRECAUTIONS – Pediatric Use).
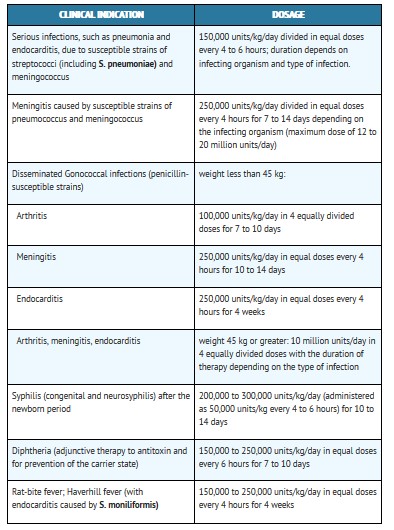
Renal Impairment
Penicillin G is relatively nontoxic, and dosage adjustments are generally required only in cases of severe renal impairment.
The recommended dosage regimens are as follows: Creatinine clearance less than 10 mL/min/1.73m2; administer a full loading dose (see recommended dosages in the tables above) followed by one-half of the loading dose every 8 to 10 hours.
Uremic patients with a creatinine clearance greater than 10 mL/min/1.73m2; administer a full loading dose (see recommended dosages in the tables above) followed by one-half of the loading dose every 4 to 5 hours.
Additional dosage modifications should be made in patients with hepatic disease and renal impairment.
For most acute infections, treatment should be continued for at least 48 to 72 hours after the patient becomes asymptomatic. Antibiotic therapy for Group A β-hemolytic streptococcal infections should be maintained for at least 10 days to reduce the risk of rheumatic fever.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Preparation of Solution
Solutions of penicillin should be prepared as follows: Loosen powder. Hold vial horizontally and rotate it while slowly directing the stream of diluent against the wall of the vial. Shake vial vigorously after all the diluent has been added. Depending on the route of administration, use Sterile Water for Injection, USP or Sterile Isotonic Sodium Chloride Solution for Parenteral use.
Note: Penicillins are rapidly inactivated in the presence of carbohydrate solutions at alkaline pH.
Reconstitution
The following table shows the amount of solvent required for solution of various concentrations:
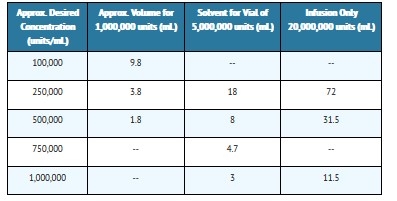
When the required volume of solvent is greater than the capacity of the vial, the penicillin can be dissolved by first injecting only a portion of the solvent into the vial, then withdrawing the resultant solution and combining it with the remainder of the solvent in a larger sterile container.
Penicillin G Potassium for Injection, USP is highly water soluble. It may be dissolved in small amounts of Water for Injection, or Sterile Isotonic Sodium Chloride Solution for Parenteral Use. All solutions should be stored in a refrigerator. When refrigerated, penicillin solutions may be stored for seven days without significant loss of potency.
Buffered Penicillin G Potassium for Injection may be given intramuscularly or by continuous intravenous drip for dosages of 500,000, 1,000,000 or 5,000,000 units. It is also suitable for intrapleural, intraarticular, and other local installations.
THE 20,000,000 UNIT (20 MILLION UNIT) DOSAGE MAY BE ADMINISTERED BY INTRAVENOUS INFUSION ONLY.
(1) Intramuscular Injection: Keep total volume of injection small. The intramuscular route is the preferred route of administration. Solutions containing up to 100,000 units of penicillin per mL of diluent may be used with a minimum of discomfort. Greater concentration of penicillin G per mL is physically possible and may be employed where therapy demands. When large doses are required, it may be advisable to administer aqueous solutions of penicillin by means of continuous intravenous drip.
(2) Continuous Intravenous Drip: Determine the volume of fluid and rate of its administration required by the patient in a 24-hour period in the usual manner for fluid therapy, and add the appropriate daily dosage of penicillin to this fluid. For example, if an adult patient requires 2 liters of fluid in 24 hours and a daily dosage of 10 million units of penicillin, add 5 million units of 1 liter and adjust the rate of flow so the liter will be infused in 12 hours.
(3) Intrapleural or Other Local Infusion: If fluid is aspirated, give infusion in a volume equal to 1/4 or 1/2 the amount of fluid aspirated, otherwise, prepare as for an intramuscular injection.
(4) Intrathecal Use: The intrathecal use of penicillin in meningitis must be highly individualized. It should be employed only with full consideration of the possible irritating effects of penicillin when used by this route. The preferred route of therapy in bacterial meningitides is intravenous, supplemented by intramuscular injection.
HOW SUPPLIED
BUFFERED PENICILLIN G POTASSIUM FOR INJECTION, USP is supplied in the following dosage forms.
NDC 51662-1436-1
BUFFERED PENICILLIN G POTASSIUM FOR INJECTION, USP 5,000,000 UNITS (5 MILLION UNITS)
HF Acquisition Co LLC, DBA HealthFirst
Mukilteo, WA 98275
Also supplied in the following manufacture supplied dosage forms
Buffered Penicillin G Potassium for Injection, USP, is supplied in dry powder form in vials containing 1,000,000 units (1 million units) × 10’s (NDC 0781-6134-95), 5,000,000 units (5 million units) × 10’s (NDC 0781-6135-95), and 20,000,000 units (20 million units) × 1’s (NDC 0781-6136-94) of crystalline penicillin G as the potassium salt; buffered with sodium citrate and citric acid to an optimum pH.
STORAGE
Store the dry powder at 20° to 25°C (68° to 77°F) [see USP controlled room temperature].
Sterile constituted solution may be kept in refrigerator 2° to 8°C (36° to 46°F) for 7 days without significant loss of potency.
REFERENCES
1.National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disk Susceptibility Tests – Fourth Edition. Tentative Standard NCCLS Document M2-T4, Vol. 8, No. 7, NCCLS, Villanova, PA, 1988.
2.
National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Second Edition. Tentative Standard NCCLS Document M7-T2, Vol. 8, No. 8. NCCLS, Villanova, PA, 1988.
3.
National Committee for Clinical Laboratory Standards, Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria – Second Edition. Tentative Standard NCCLS Document M11-T2, Villanova, PA, 1988 (or current M11-A2).
4.
1989 Sexually transmitted diseases treatment guidelines. MMWR 38(S-8); 5-14, Sept. 1, 1989.