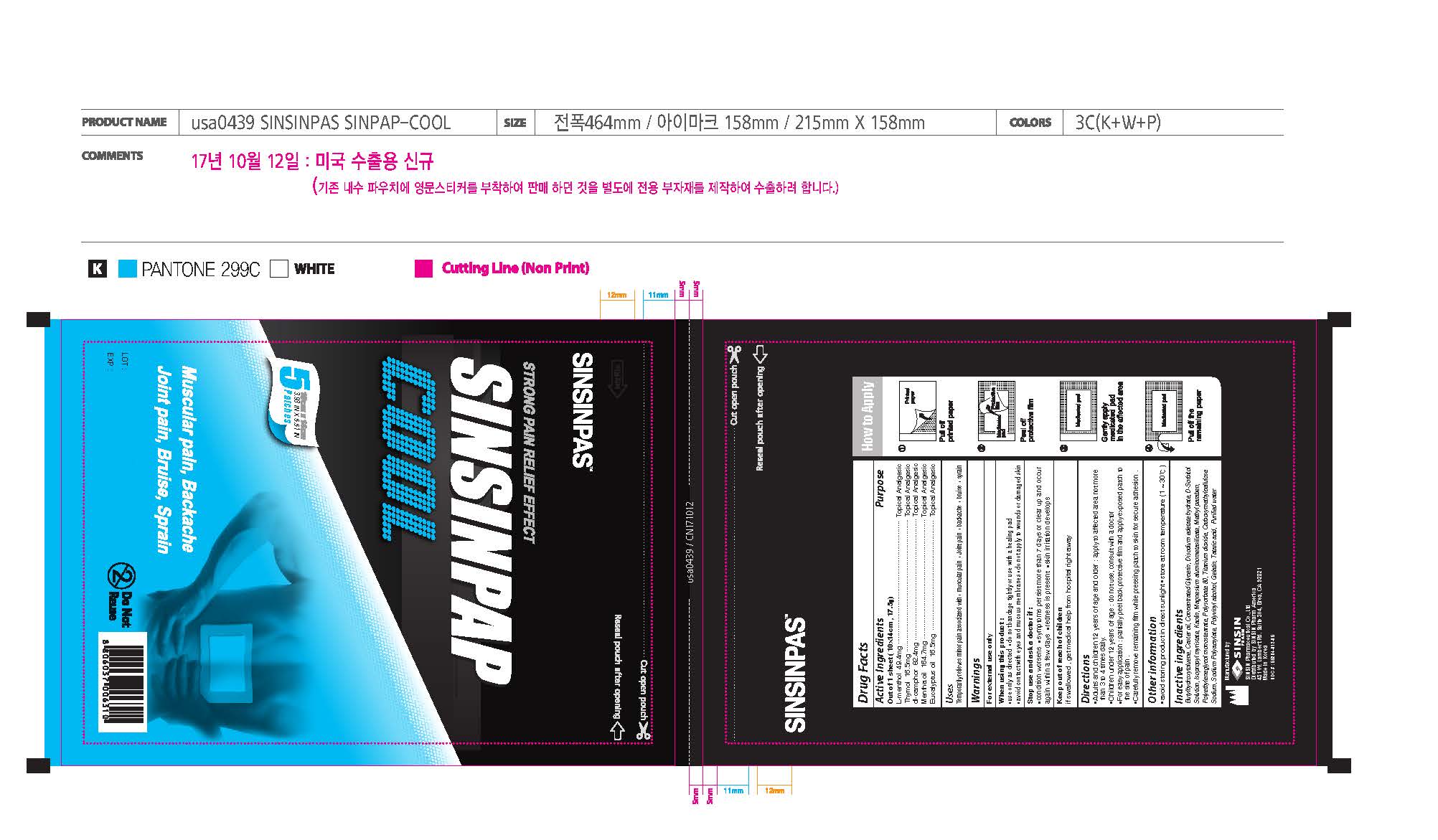
Active ingredients
L-Menthol 49.4 mg
Thymol 16.5 mg
DL-Camphor 82.4 mg
Mentha Oil 164.7 mg
Eucalyptus Oil 16.5 mg
Uses
Temporarily relieves minor pain associated with
- muscular pain
- joint pain
- backache
- bruise
- sprain
When using this product:
- use only as directed
- do not bandage tightly or use with a healing pad
- avoid contact with the eyes and mucous membranes
- do not apply to wounds or damaged skin
Stop use and ask a doctor if
- conditions worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- skin irritation develops
Directions
- Adults and children 12 years of age and over: apply to affected area not more than 3 to 4 times daily.
- Children under 12 years of age: do not use, consult a doctor
- For easy application: partially peel back protective film and apply exposed patch to the site of pain.
- Carefully remove remaining film while pressing patch to skin for secure adhesion.
Inactive ingredients
BHT, Castor oil, Concentrated Glycerin, Disodium edetate hydrate, D-sorbitol, Isopropyl myristate, Kaolin, Magnesium aluminometasilicate, Methyl Paraben, PEG-4 stearate, Polysorbate 80, Titanium dioxide, Carboxymethylcellulose Sodium, Sodium Polyacrylate, Polyvinyl alchol, Gelatin, Tataric acid, Purified water