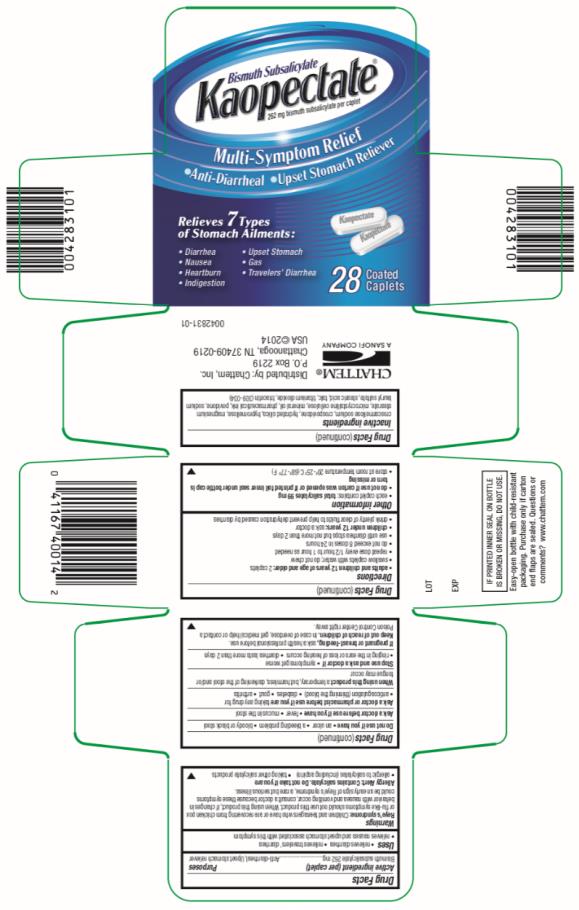
KAOPECTATE- bismuth subsalicylate tablet, film coated
Chattem, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Kaopectate Caplets
Uses
- relieves diarrhea
- relieves travelers' diarrhea
- relieves nausea and upset stomach associated with this symptom
Warnings
Reye’s Syndrome:
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy Alert: Contains salicylate.
Ask a doctor or pharmacist before use if you are
taking any drug for
- anticoagulation (thinning the blood)
- diabetes
- gout
- arthritis
Directions
- adults and children 12 years of age and older: 2 caplets
- swallow caplets with water; do not chew
- repeat does every 1/2 hour to 1 hour as needed
- do not exceed 8 doses in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
Other Information
- each caplet contains: total salicylates 99 mg
-
do not use if carton was opened or if printed foil inner seal under bottle cap is torn or missing
- store at room temperature 20º-25ºC (68º-77º F)
Inactive Ingredients
croscarmellose sodium, crospovidone, hydrated silica, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil, pharmaceutical ink, povidone, sodium lauryl sulfate, stearic acid, talc, titanium dioxide, triacetin (309-034)
Distributed by:
Chattem, Inc.
P.O. Box 2219
Chattanooga, TN
37409-0219
USA © 2013
| KAOPECTATE
bismuth subsalicylate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Chattem, Inc. (003336013) |