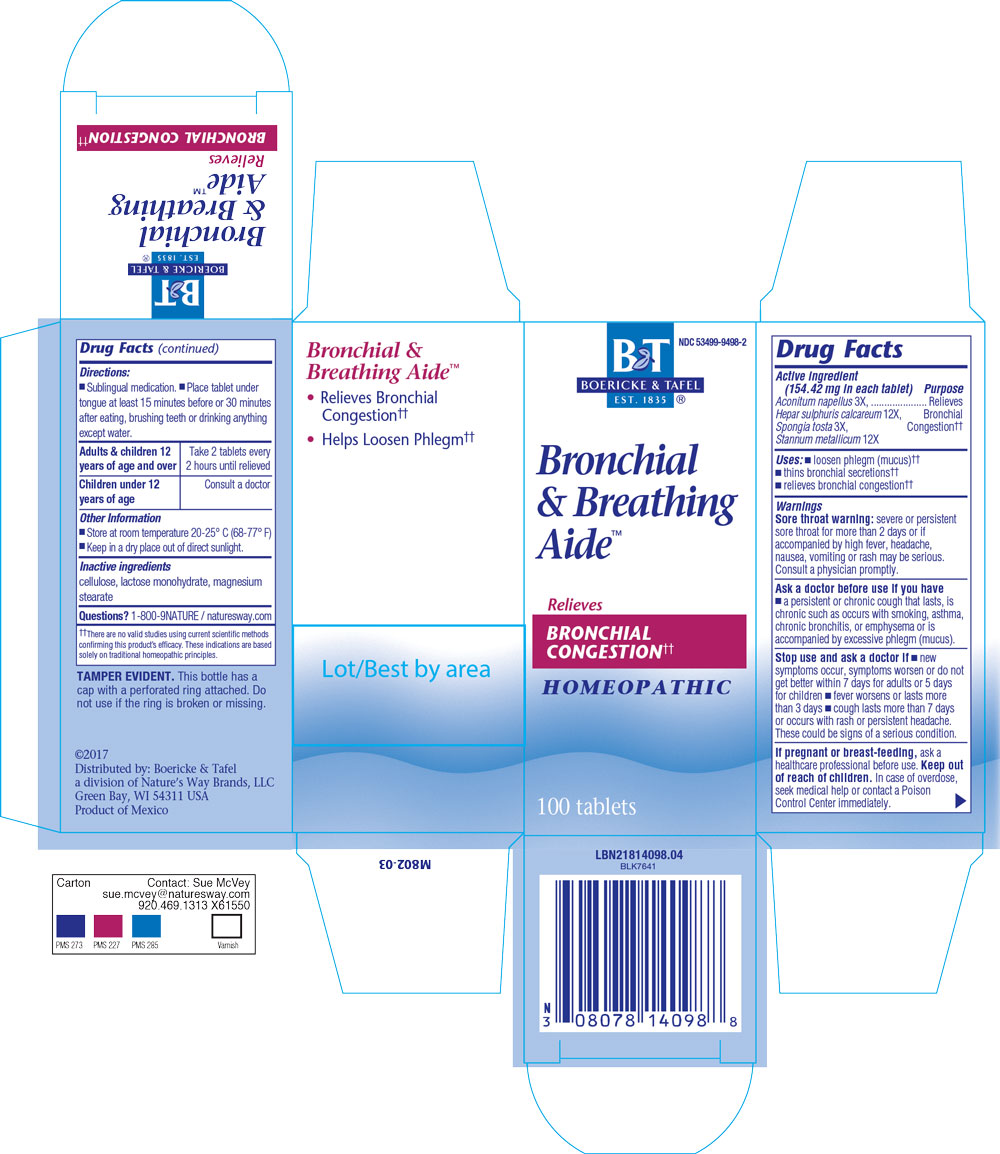
Active Ingredients
Aconitum napellus 3X
Hepar sulphuris calcareum 12X
Spongia tosta 3X
Stannum metallicum 12X
Indications & Usage
Loosen phlegm (mucus),
thins bronchial secretions,
relieves bronchial congestion
Dosage & Administration
Sublingual mediation.
Place tablet under tonge at least 15 minutes before or 30 minutes after eating, brushing teeth or drinking anything except water.
Adults & Children 12 years of age and over: Take 2 tablets every 2 hours until relieved
Children under 12 years of age: Consult a doctor
Warning
Sore Throat Warning: severe or persistent sore throat for more than 2 days or if accompanied by high fever, headache, nausea, vomiting or rash may be serious.
Consult a physician promptly.
Ask Doctor
Ask a doctor before use if you have a persistent or chronic cough that lasts, is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema or is accompanied by excessive phlegm (mucus).
Stop Use
Stop use and ask a doctor if new symptoms occur, symptoms worsen or do not get better within 7 days for adults or 5 days for children, fever worsens or lasts more than 3 days, cough lasts more than 7 days or occurs with rash or persistent headache. These could be signs of a serious condition.