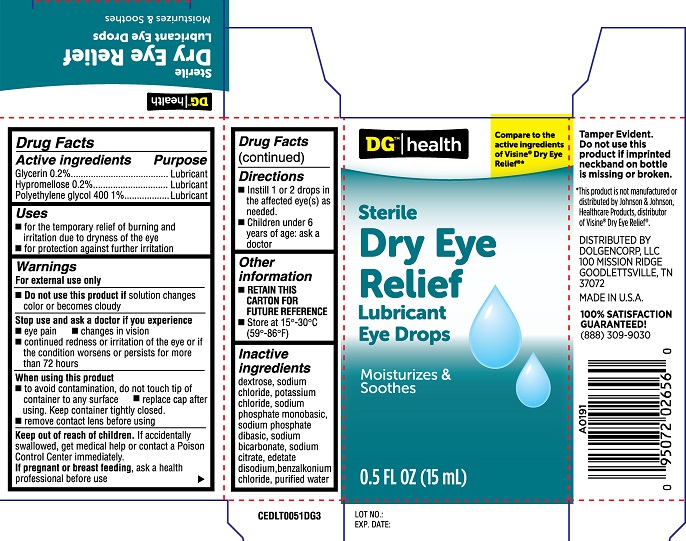
Purpose
Glycerin.............Lubricant
Hypromellose.............Lubricant
Polyethylene glycol 400.............Lubricant
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for protection against further irritation
Warnings
For external use only
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye or if the condition worsens or persists for more than 72 hours
When using this product
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using. Keep container tightly closed
- remove contact lens before using
Directions
- Instill 1 or 2 drops in the affected eye(s) as needed
- Children under 6 years of age: ask a doctor