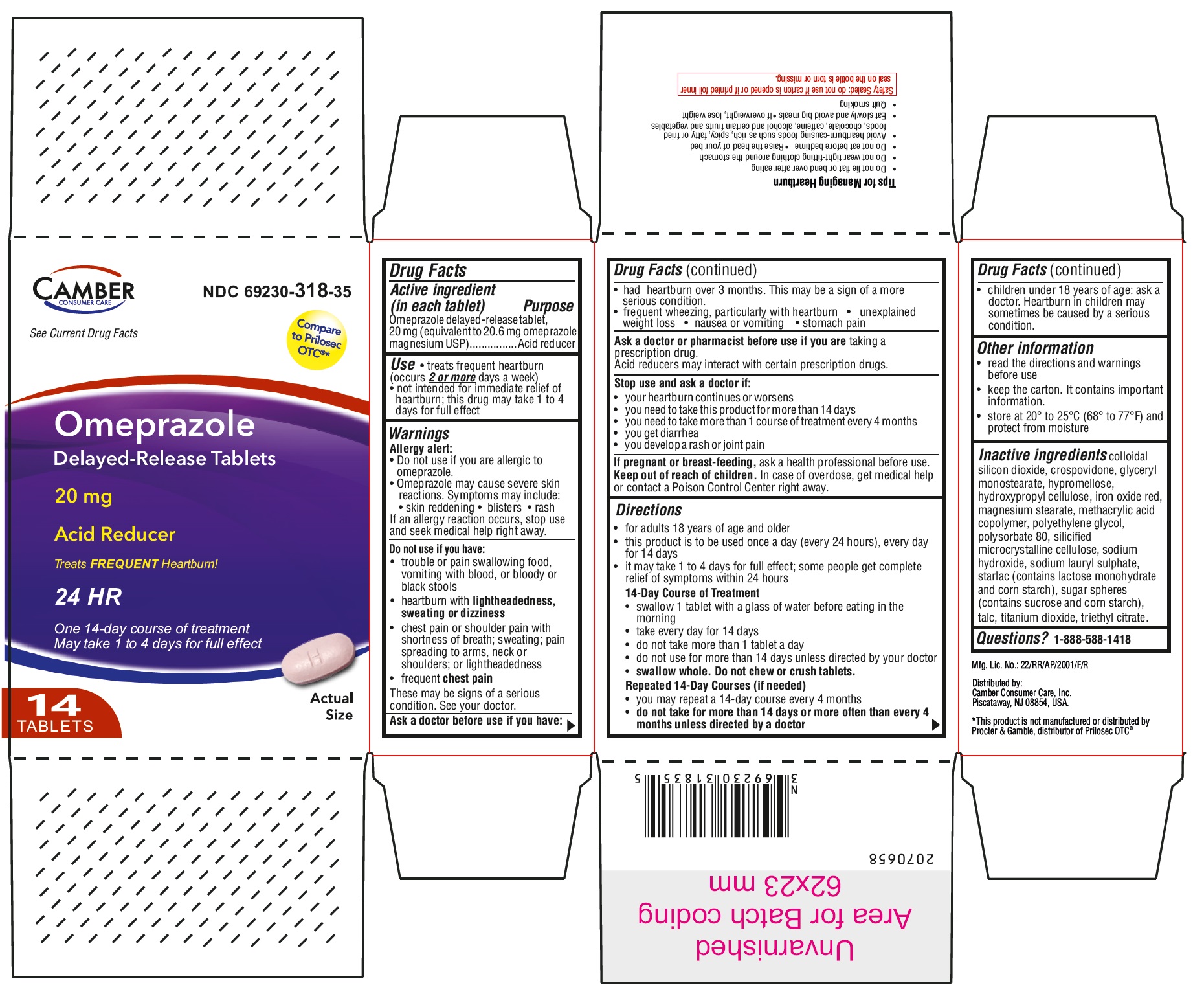
Active ingredient (in each tablet)
Omeprazole delayed-release tablet, 20 mg
(equivalent to 20.6 mg omeprazole magnesium USP)
Use
• treats frequent heartburn (occurs
2 or more days a week)
• not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
Warnings
Allergy alert:
Do not use if you are allergic to omeprazole.
• Omeprazole may cause severe skin reactions. Symptoms may include:
• skin reddening • blisters • rash
If an allergy reaction occurs, stop use and seek medical help right away.
Do not use if you have:
• trouble or pain swallowing food, vomiting with blood, or bloody or black stools
• heartburn with
lightheadedness, sweating or dizziness
• chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
• frequent
chest pain
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have:
• had heartburn over 3 months. This may be a sign of a more serious condition.
• frequent wheezing, particularly with heartburn
• unexplained weight loss • nausea or vomiting
• stomach pain
Ask a doctor or pharmacist before use if you are
taking a prescription drug.
Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if:
• your heartburn continues or worsens
• you need to take this product for more than 14 days
• you need to take more than 1 course of treatment every 4 months
• you get diarrhea
• you develop a rash or joint pain
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
• for adults 18 years of age and older
• this product is to be used once a day (every 24 hours), every day for 14 days
• it may take 1 to 4 days for full effect; some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
• swallow 1 tablet with a glass of water before eating in the morning
• take every day for 14 days
• do not take more than 14 days unless directed by your doctor
•
swallow whole. Do not chew or crush tablets.
Repeated 14-Day Courses (if needed)
• you may repeat a 14-day course every 4 months
•
do not take for more than 14 days or more often than every 4 months unless directed by a doctor
• children under 18 years of age: ask a doctor. Heartburn in children may sometimes be caused by a serious condition.
Other information
• read the directions and warnings before use
• keep the carton. It contains important information.
• store at 20° to 25° C (68° to 77° F) and protect from moisture
Inactive ingredients
colloidal silicon dioxide, crospovidone, glyceryl monostearate, hypromellose, hydroxypropyl cellulose, iron oxide red, magnesium stearate, methacrylic acid copolymer, polyethylene glycol, polysorbate 80, silicified microcrystalline cellulose, sodium hydroxide, sodium lauryl sulphate, starlac (contains lactose monohydrate and corn starch), sugar spheres (contains sucrose and corn starch), talc, titanium dioxide, triethyl citrate.