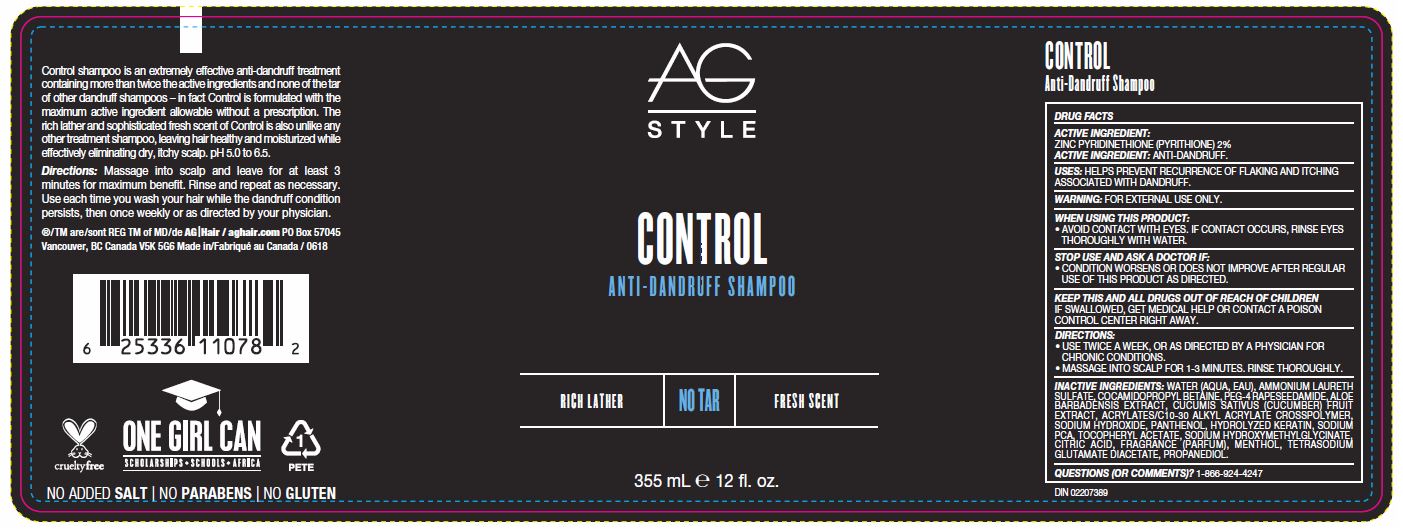
STOP USE AND ASK A DOCTOR IF:
CONDITION WORSENS OR DOES NOT IMPROVE AFTER REGULAR USE OF THIS PRODUCT AS DIRECTED.
KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDREN
IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS:
- USE TWICE A WEEK, OR AS DIRECTED BY A PHYSICIAN FOR CHRONIC CONDITIONS.
- MASSAGE INTO SCALP FOR 3-5 MINUTES. RINSE THOROUGHLY.
INACTIVE INGREDIENTS
WATER (AQUA, EAU), AMMONIUM LAURETH SULFATE, COCAMIDOPROPYL BETAINE, PEG-4 RAPESEEDAMIDE, ALOE BARBADENSIS EXTRACT, CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, SODIUM HYDROXIDE, PANTHENOL, HYDROLYZED KERATIN, SODIUM PCA, TOCOPHERYL ACETATE, SODIUM HYDROXYMETHYLGLYCINATE, CITRIC ACID, FRAGRANCE (PARFUM), ENTHOL, TETRASODIUM GLUTAMATE DIACETATE, PROPANEDIOL.