ITCH RELIEF- apis mellifera, lachesis muta venom, ledum palustre twig and urtica urens liquid
Similasan AG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredient
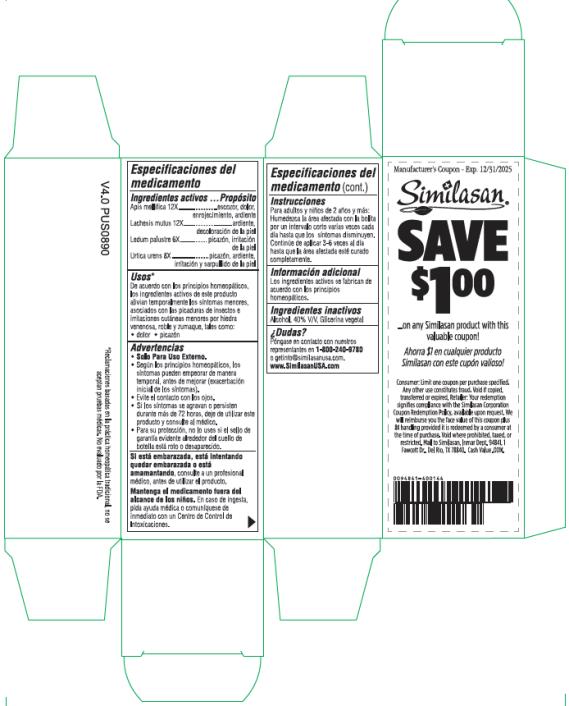
Apis mellifica 12X
Purpose
stinging, pain, redness, burning
Active Ingredient
Lachesis mutus 12X
Purpose
burning, skin discoloration
Active Ingredient
Ledum palustre 6X
Purpose
itching, skin irritation
Active Ingredient
Urtica urens 8X
Purpose
itching, burning, skin irritation and rashes
Uses:
According to homeopathic principles, the active ingredients in this product temporarily relieve minor symptoms associated with insect bites & stings and minor skin irritations due to poison ivy, oak & sumac such as: pain itching
Warnings:
-
For external use only.
- According to homeopathic principles, symptoms may temporarily worsen before improving (initial exacerbation of symptoms).
- Avoid contact with the eyes.
- If conditions worsen or persist for more than 72 hours discontinue use and consult a physician.
If pregnant, trying to get pregnant or breast feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
For adults and children age 2 and over: Moisten the affected area with roller ball at short intervals several times a day until the symptoms clearly diminish. Continue to apply 3-6 times daily until the affected area is completely cleared up.
Other Information:
Active ingredients are manufactured according to homeopathic principles.
Inactive Ingredients:
Alcohol, 40% V/V, Vegetable glycerin
Questions?
Reach our representatives at 1-800-240-9780 or getinfo@similasanusa.com.
www.SimilasanUSA.com
PRINCIPAL DISPLAY PANEL
Similasan®
Itch Relief
ROLL-ON
7.5 mL/0.25 fl oz