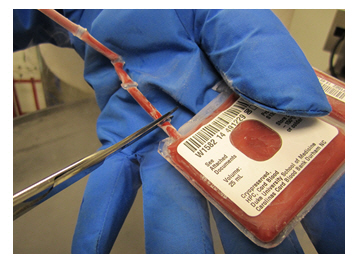
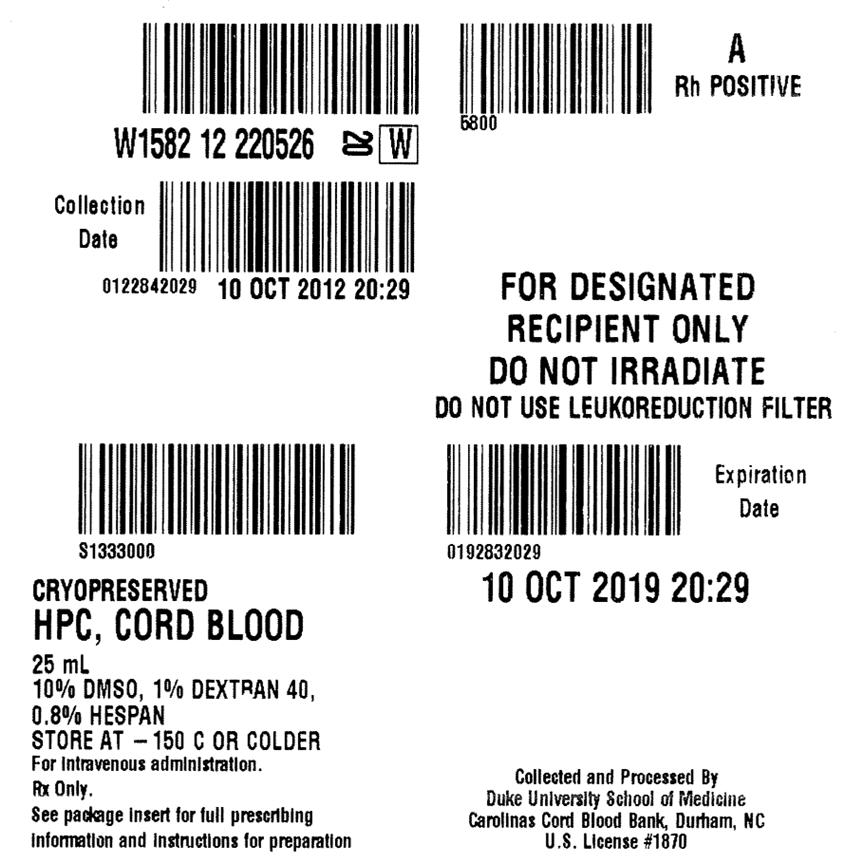
FULL PRESCRIBING INFORMATION
WARNING: FATAL INFUSION REACTIONS, GRAFT VERSUS HOST DISEASE, ENGRAFTMENT SYNDROME, AND GRAFT FAILURE
Fatal infusion reactions: DUCORD administration can result in serious, including fatal, infusion reactions. Monitor patients and discontinue DUCORD infusion for severe reactions. [See Warnings and Precautions (5.1, 5.2)]
Graft-vs-host disease (GVHD): GVHD is expected after administration of DUCORD, and may be fatal. Administration of immunosuppressive therapy may decrease the risk of GVHD. [See Warnings and Precautions (5.3)]
Engraftment syndrome: Engraftment syndrome may progress to multiorgan failure and death. Treat engraftment syndrome promptly with corticosteroids. [See Warnings and Precautions (5.4)]
Graft failure: Graft failure may be fatal. Monitor patients for laboratory evidence of hematopoietic recovery. Prior to choosing a specific unit of DUCORD, consider testing for HLA antibodies to identify patients who are alloimmunized. [See Warnings and Precautions (5.5)]
1 INDICATIONS AND USAGE
DUCORD, HPC (Hematopoietic Progenitor Cell), Cord Blood, is an allogeneic cord blood hematopoietic progenitor cell therapy indicated for use in unrelated donor hematopoietic progenitor stem cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment.
The risk benefit assessment for an individual patient depends on the patient characteristics, including disease, stage, risk factors, and specific manifestations of the disease, on characteristics of the graft, and on other available treatments or types of hematopoietic progenitor cells.
2 DOSAGE AND ADMINISTRATION
- For intravenous use only.
- Do not irradiate.
Unit selection and administration of DUCORD should be done under the direction of a physician experienced in hematopoietic progenitor cell transplantation.
2.1 Dosing
The recommended minimum dose is 2.5 × 107 nucleated cells/kg at cryopreservation. Multiple units may be required in order to achieve the appropriate dose.
Matching for at least 4 of 6 HLA-A antigens, HLA-B antigens, and HLA-DRB1 alleles is recommended. The HLA typing and nucleated cell content for each individual unit of DUCORD are documented on the container label and/or in accompanying records.
2.2 Preparation for Infusion
DUCORD should be prepared by a trained healthcare professional.
- Do not irradiate DUCORD.
- See the appended detailed instructions for preparation of DUCORD for infusion.
- Once prepared for infusion, DUCORD may be stored at 4 to 8 °C for up to 30 minutes if product is thawed and DMSO is not removed, at 4 to 8 °C for up to 1 hour if DUCORD is thawed and diluted to reduce DMSO concentration, and at 4 to 8 °C for up to 4 hours if DMSO is removed in a washing procedure [see Instructions for Preparation for Infusion].
- The recommended limit on DMSO administration is 1 gram per kg body weight per day. [See Warnings and Precautions (5.2) and Overdosage (10 ]
2.3 Administration
DUCORD should be administered under the supervision of a qualified healthcare professional experienced in hematopoietic progenitor cell transplantation.
- Confirm the identity of the patient for the specified unit of DUCORD prior to administration.
- Confirm that emergency medications are available for use in the immediate area.
- Ensure the patient is hydrated adequately.
- Premedicate the patient 30 to 60 minutes before the administration of DUCORD. Premedication should include any or all of the following: antipyretic, histamine antagonists, and corticosteroids.
- Inspect the product for any abnormalities, such as unusual particulates, and for breaches of container integrity prior to administration. Prior to infusion, discuss all such product irregularities with the laboratory issuing the product for infusion.
- Administer DUCORD by intravenous infusion. Do not administer in the same tubing concurrently with products other than 0.9% Sodium Chloride, Injection (USP). DUCORD may be filtered through a 170 to 260 micron filter designed to remove clots. Do NOT use a filter designed to remove leukocytes.
- DUCORD should be infused over 15 to 60 minutes depending on the volume of the product and the weight of the patient. The rate of infusion should not exceed a maximum of 5 milliliters per kilogram per hour. The infusion rate should be reduced if the fluid load is not tolerated. The infusion should be discontinued in the event of an allergic reaction or if the patient develops a moderate to severe infusion reaction. [See Warnings and Precautions (5.2) and Adverse Reactions (6)]
- Monitor the patient for adverse reactions during, and for at least six hours after, administration. Because DUCORD contains lysed red cells that may cause renal failure, careful monitoring of urine output is also recommended.
NOTE: If product is being prepared for a multi-unit infusion, infuse units independently.
Should a reaction occur, appropriately manage the reaction before the second unit is thawed for infusion.
3 DOSAGE FORMS AND STRENGTHS
Each unit of DUCORD contains a minimum of 9.0 × 108 total nucleated cells with a minimum of 1.25 × 106 viable CD34+ cells, suspended in 10% dimethyl sulfoxide (DMSO) and 1% Dextran 40, at the time of cryopreservation.
The exact pre-cryopreservation nucleated cell content is provided on the container label and in accompanying records.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Allergic reactions may occur with infusion of HPC, Cord Blood, including DUCORD. Reactions include bronchospasm, wheezing, angioedema, pruritus, and hives [see Adverse Reactions (6)]. Serious hypersensitivity reactions, including anaphylaxis, also have been reported. These reactions may be due to dimethyl sulfoxide (DMSO), Dextran 40, hydroxyethylstarch, or a plasma component of DUCORD.
DUCORD may contain residual antibiotics if the cord blood donor was exposed to antibiotics in utero. Patients with a history of allergic reactions to antibiotics should be monitored for allergic reactions following DUCORD administration.
5.2 Infusion Reactions
Infusion reactions are expected to occur and include nausea, vomiting, fever, rigors or chills, flushing, dyspnea, hypoxemia, chest tightness, hypertension, tachycardia, bradycardia, dysgeusia, hematuria, and mild headache. Premedication with antipyretic, histamine antagonists, and corticosteroids may reduce the incidence and intensity of infusion reactions.
Severe reactions, including respiratory distress, severe bronchospasm, severe bradycardia with heart block or other arrhythmias, cardiac arrest, hypotension, hemolysis, elevated liver enzymes, renal compromise, encephalopathy, loss of consciousness, and seizure also may occur. Many of these reactions are related to the amount of DMSO administered. Minimizing the amount of DMSO administered may reduce the risk of such reactions, although idiosyncratic responses may occur even at DMSO doses thought to be tolerated. The actual amount of DMSO depends on the method of preparation of the product for infusion. Limiting the amount of DMSO infused to no more than 1 gram per kilogram per day is recommended. [See Overdosage (10)]
Infusion reactions may begin within minutes of the start of infusion of DUCORD, although symptoms may continue to intensify and not peak for several hours after completion of the infusion. Monitor the patient closely during this period. When a reaction occurs, discontinue the infusion and institute supportive care as needed.
If infusing more than one unit of DUCORD on the same day, do not administer subsequent units until all signs and symptoms of infusion reactions from the prior unit have resolved.
5.3 Graft-versus-Host Disease
Acute and chronic graft-versus-host disease (GVHD) may occur in patients who have received DUCORD. Classic acute GVHD is manifested as fever, rash, elevated bilirubin and liver enzymes, and diarrhea. Patients transplanted with DUCORD also should receive immunosuppressive drugs to decrease the risk of GVHD. [See Adverse Reactions (6.1)]
5.4 Engraftment Syndrome
Engraftment syndrome is manifested as unexplained fever and rash in the peri-engraftment period. Patients with engraftment syndrome also may have unexplained weight gain, hypoxemia, and pulmonary infiltrates in the absence of fluid overload or cardiac disease. If untreated, engraftment syndrome may progress to multiorgan failure and death. Once engraftment syndrome is recognized, begin treatment with corticosteroids to ameliorate the symptoms. [See Adverse Reactions (6.1)]
5.5 Graft Failure
Primary graft failure, which may be fatal, is defined as failure to achieve an absolute neutrophil count greater than 500 per microliter blood by Day 42 after transplantation. Immunologic rejection is the primary cause of graft failure. Patients should be monitored for laboratory evidence of hematopoietic recovery. Consider testing for HLA antibodies in order to identify patients who are alloimmunized prior to transplantation and to assist with choosing a unit with a suitable HLA type for the individual patient. [See Adverse Reactions (6.1)]
5.6 Malignancies of Donor Origin
Patients who have undergone HPC, Cord Blood transplantation may develop post-transplant lymphoproliferative disorder (PTLD), manifested as a lymphoma-like disease favoring nonnodal sites. PTLD is usually fatal if not treated.
The incidence of PTLD appears to be higher in patients who have received antithymocyte globulin. The etiology is thought to be donor lymphoid cells transformed by Epstein-Barr virus (EBV). Serial monitoring of blood for EBV DNA may be warranted in high-risk groups.
Leukemia of donor origin also has been reported in HPC, Cord Blood recipients. The natural history is presumed to be the same as that for de novo leukemia.
5.7 Transmission of Serious Infections
Transmission of infectious disease may occur because DUCORD is derived from human blood. Disease may be caused by known or unknown infectious agents. Donors are screened for increased risk of infection with human immunodeficiency virus (HIV), human T-cell lymphotropic virus (HTLV), hepatitis B virus (HBV), hepatitis C virus (HCV), T. pallidum, T. cruzi, West Nile Virus (WNV), transmissible spongiform encephalopathy (TSE) agents, and vaccinia. Donors are also screened for clinical evidence of sepsis, and communicable disease risks associated with xenotransplantation. Maternal blood samples are tested for HIV types 1 and 2, HTLV types I and II, HBV, HCV, T. pallidum, WNV, and T. cruzi. DUCORD is tested for sterility. There may be an effect on the reliability of the sterility test results if the cord blood donor was treated with antibiotics. These measures do not totally eliminate the risk of 6 of 30 transmitting these or other transmissible infectious diseases and disease agents. Report the occurrence of a transmitted infection to Duke University School of Medicine, Carolinas Cord Blood Bank at 1-919-668-1102.
Testing is also performed for evidence of donor infection due to cytomegalovirus (CMV).
Test results may be found in accompanying records.
5.8 Transmission of Rare Genetic Diseases
DUCORD may transmit rare genetic diseases involving the hematopoietic system for which donor screening and/or testing has not been performed [see Adverse Reactions (6.1)]. Cord blood donors have been screened by family history to exclude inherited disorders of the blood and marrow. DUCORD has been tested to exclude donors with sickle cell anemia, and anemias due to abnormalities in hemoglobins C, D, and E. Because of the age of the donor at the time DUCORD collection takes place, the ability to exclude rare genetic diseases is severely limited.
6 ADVERSE REACTIONS
Day-100 mortality from all causes was 25%.
The most common infusion-related adverse reactions (≥5%) are hypertension, vomiting, nausea, bradycardia, and fever.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety assessment of DUCORD is based primarily on review of the data submitted to the FDA dockets from various sources, the dataset for the COBLT Study, and published literature.
Infusion Reactions
The data described in Table 1 reflect exposure to 442 infusions of HPC, Cord Blood (from multiple cord blood banks) in patients treated using a total nucleated cell dose ≥2.5 × 107/kg on a single-arm trial or expanded access use (The COBLT Study). The population was 60% male and the median age was 5 years (range 0.05-68 years), and included patients treated for hematologic malignancies, inherited metabolic disorders, primary immunodeficiencies, and bone marrow failure. Preparative regimens and graft-vs-host disease prophylaxis were not standardized. The most common infusion reactions were hypertension, vomiting, nausea, and sinus bradycardia. Hypertension and any grades 3-4 infusion-related reactions occurred more frequently in patients receiving HPC, Cord Blood in volumes greater than 150 milliliters and in pediatric patients. The rate of serious adverse cardiopulmonary reactions was 0.8%.
| Any grade | Grade 3-4 | |
|---|---|---|
| Any reaction | 65.4% | 27.6% |
| Hypertension | 48.0% | 21.3% |
| Vomiting | 14.5% | 0.2% |
| Nausea | 12.7% | 5.7% |
| Sinus bradycardia | 10.4% | 0 |
| Fever | 5.2% | 0.2% |
| Sinus tachycardia | 4.5% | 0.2% |
| Allergy | 3.4% | 0.2% |
| Hypotension | 2.5% | 0 |
| Hemoglobinuria | 2.1% | 0 |
| Hypoxia | 2.0% | 2.0% |
Information on infusion reactions was available from voluntary reports for 388 infusions for patients who received DUCORD at a total nucleated cell dose of ≥2.5×107/kg. The population included 57% males and 43% females with median age of 16 years (range 0.1-79 years). Preparative regimens and graft-vs-host disease prophylaxis were not standardized. The reactions were not graded. Nineteen percent of infusions (n=72) were associated with an infusion reaction. The most common infusion reactions, occurring in >1% of infusions, were hypertension (15%), nausea or vomiting (3.4%), bradycardia (1.8%), and chest pain (1.3%).
Other Adverse Reactions
For other adverse reactions, the raw clinical data from the docket were pooled for 1299 (120 adult and 1179 pediatric) patients transplanted with HPC, Cord Blood (from multiple cord blood banks) with total nucleated cell dose ≥2.5 × 107/kg. Of these, 66% (n=862) underwent transplantation as treatment for hematologic malignancy. The preparative regimens and graft-vshost disease prophylaxis varied. The median total nucleated cell dose was 6.4 × 107/kg (range, 2.5-73.8 107/kg). For these patients, Day-100 mortality from all causes was 25%. Primary graft failure occurred in 16%; 42% developed grades 2-4 acute graft-vs-host disease; and 19% developed grades 3-4 acute graft-vs-host disease.
Data from published literature and from observational registries, institutional databases, and cord blood bank reviews reported to the docket for HPC, Cord Blood (from multiple cord blood banks) revealed nine cases of donor cell leukemia, one case of transmission of infection, and one report of transplantation from a donor with an inheritable genetic disorder. The data are not sufficient to support reliable estimates of the incidences of these events.
In the COBLT Study, 15% of the patients developed engraftmentsyndrome.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with DUCORD use in pregnant women to inform a product-associated risk. Animal reproduction studies have not been conducted with DUCORD. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of DUCORD in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DUCORD and any potential adverse effects on the breastfed infant from DUCORD or from the underlying maternal condition.
8.4 Pediatric Use
HPC, Cord Blood has been used in pediatric patients with disorders affecting the hematopoietic system that are inherited, acquired, or resulted from myeloablative treatment. [See Dosage and Administration (2), Adverse Reactions (6), and Clinical Studies (14)]
8.5 Geriatric Use
Clinical studies of HPC, Cord Blood (from multiple cord blood banks) did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. In general, administration of DUCORD to patients over age 65 should be cautious, reflecting their greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
10.1 Human Overdosage Experience
There has been no experience with overdosage of HPC, Cord Blood in human clinical trials. Single doses of DUCORD up to 9.1 × 108 TNC/kg have been administered. HPC, Cord Blood prepared for infusion may contain dimethyl sulfoxide (DMSO). The maximum tolerated dose of DMSO has not been established, but it is customary not to exceed a DMSO dose of 1 gm/kg/day when given intravenously. Several cases of altered mental status and coma have been reported with higher doses of DMSO.
11 DESCRIPTION
DUCORD consists of hematopoietic progenitor cells, monocytes, lymphocytes, and granulocytes from human cord blood for intravenous infusion. Blood recovered from umbilical cord and placenta is volume reduced and partially depleted of red blood cells and plasma.
The active ingredient is hematopoietic progenitor cells which express the cell surface marker CD34. The potency of cord blood is determined by measuring the numbers of total nucleated cells (TNC) and CD34+ cells, and cell viability. Each unit of DUCORD contains a minimum of 9 × 108 total nucleated cells with at least 1.25 × 106 viable CD34+ cells at the time of cryopreservation. The cellular composition of DUCORD depends on the composition of cells in the blood recovered from the umbilical cord and placenta of the donor. The actual nucleated cell count, the CD34+ cell count, the ABO group, and the HLA typing are listed on the container label and/or accompanying records sent with each individual unit.
DUCORD has the following inactive ingredients: dimethyl sulfoxide (DMSO), citrate phosphate dextrose (CPD), hydroxyethylstarch, and Dextran 40. When prepared for infusion according to instructions, the infusate contains the following inactive ingredients: Dextran 40, human serum albumin, residual DMSO, and CPD.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Hematopoietic stem/progenitor cells from HPC, Cord Blood migrate to the bone marrow where they divide and mature. The mature cells are released into the bloodstream, where some circulate and others migrate to tissue sites, partially or fully restoring blood counts and function, including immune function, of blood-borne cells of marrow origin. [See Clinical Studies (14)]
In patients with enzymatic abnormalities due to certain severe types of storage disorders, mature leukocytes resulting from HPC, Cord Blood transplantation may synthesize enzymes that may be able to circulate and improve cellular functions of some native tissues. However, the precise mechanism of action is unknown.
14 CLINICAL STUDIES
The effectiveness of DUCORD, as defined by hematopoietic reconstitution, was demonstrated in one single-arm prospective study, and in retrospective reviews of data from an observational database for DUCORD and data in the dockets and public information. Of the 1299 patients in the dockets and public data, 66% (n=862) underwent transplantation as treatment for hematologic malignancy. Results for patients who received a total nucleated cell dose ≥2.5 × 107/kg are shown in Table 2. Neutrophil recovery is defined as the time from transplantation to an absolute neutrophil count more than 500 per microliter. Platelet recovery is the time to a platelet count more than 20,000 per microliter. Erythrocyte recovery is the time to a reticulocyte count greater than 30,000 per microliter. The total nucleated cell dose and degree of HLA match were inversely associated with the time to neutrophil recovery in the docket data.
| Data Source | The COBLT Study* | Docket* and Public Data* | DUCORD |
|---|---|---|---|
| Design | Single-arm prospective | Retrospective | Retrospective |
| Number of patients | 324 | 1299 | 550 |
| Median age (range) | 4.6 (0.07 – 52.2) yrs | 7.0 (<1 – 65.7) yrs | 11 (0.083 – 79) yrs |
| Gender | 59% male 41% female | 57% male 43% female | 56% male 44% female |
| Median TNC Dose (range) (× 107/kg) | 6.7 (2.6 – 38.8) | 6.4 (2.5 – 73.8) | 6.6 (2.5 – 58) |
| Neutrophil Recovery at Day 42 (95% CI) | 76% (71% – 81%) | 77% (75% – 79%) | 95%† (92% – 96%) |
| Platelet Recovery at Day 100 (20,000/uL) (95% CI) | 57% (51% – 63%) | - | 92%† (89% – 96%) |
| Platelet Recovery at Day 100 (50,000/uL) (95% CI) | 46% (39% – 51%) | 45% (42% – 48%) | 71%† (66% – 75%) |
| Erythrocyte Recovery at Day 100 (95% CI) | 65% (58% – 71%) | - | - |
| Median time to Neutrophil Recovery | 27 days | 25 days | 21 days† |
| Median time to Platelet Recovery (20,000/uL) | 90 days | - | 46 days† |
| Median time to Platelet Recovery (50,000/uL) | 113 days | 122 days | 61 days† |
| Median time to Erythrocyte Recovery | 64 days | - | - |
16 HOW SUPPLIED/STORAGE AND HANDLING
DUCORD is supplied as a cryopreserved cell suspension in a sealed bag containing a minimum of 9 × 108 total nucleated cells with a minimum of 1.25 × 106 viable CD34+ cells in a volume of 25 milliliters (ISBT 128, Product Code S1333000, ISBT 128 Facility Identifier Number W1582). The exact pre-cryopreservation nucleated cell content is provided on the container label and accompanying records.
DUCORD units manufactured prior to 01-06-2014 are supplied in a cryopreservation bag with up to 3 attached segments and spike ports that are compatible with the Pall Cell wash/infusion bag set (see section I in Instructions for Preparation for Infusion below). DUCORD units manufactured on and after 01-06-2014 are supplied in the Biosafe 4b bag, which contains up to 4 attached segments and slightly modified spike ports. The 4b bag must be spiked with the Biosafe 4b coupler, which can be heat-sealed to the Pall Cell wash/infusion bag set (see section II,3 in Instructions for Preparation for Infusion below). The year of manufacture is noted in the ISBT number (W1582 14…, for manufacture in 2014), and the specific date of manufacture can be found on page 3 of the NMDP CBU Detail Report.
17 PATIENT COUNSELING INFORMATION
Discuss the following with patients receiving DUCORD:
- Report immediately any signs and symptoms of acute infusion reactions, such as fever, chills, fatigue, breathing problems, dizziness, nausea, vomiting, headache, or muscle aches.
- Report immediately any signs or symptoms suggestive of graft-vs-host disease, including rash, diarrhea, or yellowing of the eyes.
INSTRUCTIONS FOR PREPARATION FOR INFUSION
- I
-
MATERIALS AND EQUIPMENT
- 1
-
Materials
- Albumin (human) 25%, USP
- Dextran 40 in Sodium Chloride Injection, USP (10% Low Molecular Weight Dextran in 0.9% Sodium Chloride Injection)
- 0.4% Trypan Blue vital stain solution
- 0.9% Sodium Chloride Injection, USP (normal saline solution)
- Cell wash/infusion bag set (Pall Medical Cell Wash/Infusion Bag Set or equivalent)
- Coupler spike set (Biosafe for 4b cryobag) for CBUs manufactured on or after 01-06-2014.
- 150 and 300 mL transfer bags
- Sampling site coupler
- 3, 20, 30 and 60 mL sterile disposable syringes
- 16 gauge injection needles
- Alcohol cleaned scissors
- 5 mL sterile culture tubes (snap cap)
- 5 mL polystyrene tubes
- 2 mL Cryogenic vials (Nunc or equivalent)
- Alcohol prep pads and/or isopropyl alcohol
- Iodine swab sticks
- 7 × 8 inch sterile zip-lock bags (or similar size)
- Hemostats (optional)
- Tape
- Gloves
- Protective freezer gloves
- Reusable ice mats (Insul-Ice Mat or equivalent)
- 4 × 4 inch sterile gauze pads (or similar size)
- 250 mL blood bank centrifuge insert (or similar size)
- 100 mL burette hemoset 170-260 micron filter
- 2-gang, 3-way stopcocks with male-luer-lock (or equivalent)
- Lipid compatible 3-way stopcock
- Rubber-shod clamp
- 60 inch, 2.4 mL extension set tubing (APV or equivalent)
- Neutral displacement needleless connector (ICU Medical MicroCLAVE EndCap or equivalent)
- 4 – 5 inch sterile spinal needle or other sterilized blunt needle-like device with luer-lock tip
- Product labels (ISBT [International Society of Blood Transfusion] or equivalent)
- Forms:
- Site specific forms generated to capture processing data and to accompany the infusion product to the bedside
- Cord Blood Unit (CBU) Shipment Packing Information Form: to acknowledges Product ID and shipping information. This form is retained at the transplant center
- National Marrow Donor Program (NMDP) Receipt of Cord Blood Unit Form: completed by transplant center and return to Duke University School of Medicine, Carolinas Cord Blood Bank (CCBB)
- 2
-
Equipment
- Biological Safety Cabinet
- Refrigerated blood bank centrifuge (Sorvall or equivalent)
- Buckets and supports (inserts) to centrifuge blood bags in the refrigerated blood bank centrifuge
- Plasma extractor or expressor
- Scale (with Plexiglass tray or equivalent)
- Sterile tubing welder device
- Tube heat sealer for polyvinyl chloride (PVC) plastic
- Automated cell counter
- Optical microscope
- Vortex mixer
- Water bath (4 liters or more at 37°C)
- Thermogenesis canister opener
- Non frost-free refrigerator
- Transport container (validated to maintain temperature between 4-8°C)
- Tube rack
- Vapor phase liquid nitrogen (LN2) freezer
- II
-
PREPARATION
- 1
-
General Preparation Information
- a.
- Use aseptic technique in a biological safety cabinet for all processing steps, including all open-container processing and all spiking of blood bag ports.
- b.
- Use only sterile materials when processing the cellular product.
- c.
- Record the manufacturer information, lot number and expiration date (if applicable) of all reagents and disposables.
- d.
- For CBUs manufactured on or after 01-06-2014 in the Biosafe 4b cryobag, replace spike ports to cryobag with Biosafe 4b coupler kit (included in shipment) per steps in Section II.3 below.
- e.
- Pre-cool Dextran 40 solution to 4-8°C in refrigerator before mixing with human albumin to prepare the thawing solution.
- f.
- Verify the water bath is full and that the temperature is 37°C.
- g.
- Verify the centrifuge is set at 2-8ºC.
- h.
- Keep thawed product at 2-8ºC throughout the procedure.
- i.
- Schedule the infusion time of the transplant with the transplant team in advance of the procedure. Re-confirm on the day of infusion with the transplant team so that the start time for the thawing procedure can be adjusted to have the unit ready for infusion at a time the patient can receive the infusion.
- j.
- Confirm the final product volume required for infusion (based on the patient's weight or not more than 60 mL for the Syringe Method) with the transplant team prior to thawing DUCORD. Record on appropriate laboratory form.
- k.
- Read section VIII EMERGENCY PRODUCT RECOVERY IN THE EVENT OF A CONTAINER FAILURE prior to thawing product. Always thaw and centrifuge the cryobag and or infusion bag inside of a sterile zip-lock bag so that the product can be recovered if leakage occurs. If the product leaks from the cryobag into the sterile zip-lock bag, use the product recovery process described below in section VIII.
- l.
- Contact the CCBB Laboratory Supervisor at 919-257-9273 or 919-668-2066 if during thawing, any portion of the product or container appears to be damaged or compromised. The CCBB will provide advice to determine the best way to recovery the product from the compromised container. The CCBB will also provide further instructions for return of the container, if requested, and a prompt investigation will follow. The transplant physician and the local laboratory director should also be notified immediately.
- 2
-
Prepare Dextran 40/Albumin Solution (Thawing Solution)
- a.
- Use sterile technique to connect an empty 300 mL transfer bag to a 500 mL bag of Dextran 40 solution.
- b.
- Place the empty transfer bag on the scale and tare the scale.
- c.
- Transfer 150 grams of pre-cooled Dextran 40 solution to the 300 mL transfer bag.
- d.
- Heat seal tubing and detach Dextran 40 solution bag from the 300 mL transfer bag by cutting tubing at the sealed point leaving sufficient tubing length for sterile welding later. (See step 3b below)
- e.
- Insert a sampling site coupler into one of the ports of the 300 mL transfer bag containing the Dextran 40 solution.
- f.
- Clean the rubber stopper of human albumin bottle with alcohol wipes.
- g.
- Use a 30 mL syringe to draw up 30 mL of 25% human albumin.
- h.
- Clean coupler port with alcohol and inject 30 mL of human albumin into the transfer bag containing 150 mL of Dextran 40 solution.
NOTE: The transfer bag now contains a total of approximately 180 mL of solution containing both Dextran 40 and human albumin and will be referred to as the Dextran 40/Albumin bag in subsequent steps. The solution in this bag (with the final concentration of 8.3% Dextran 40/4.2 % Albumin) will be referred to as the thawing solution.
- 3
-
Assemble the Closed System
- a.
- Clamp all tubing and place assigned labels on the cell wash/infusion bag set (cell/wash infusion bag attached to supernatant bag) (see Figure 1).
NOTE: The cell wash/infusion bag will be referred to as infusion bag in subsequent steps.
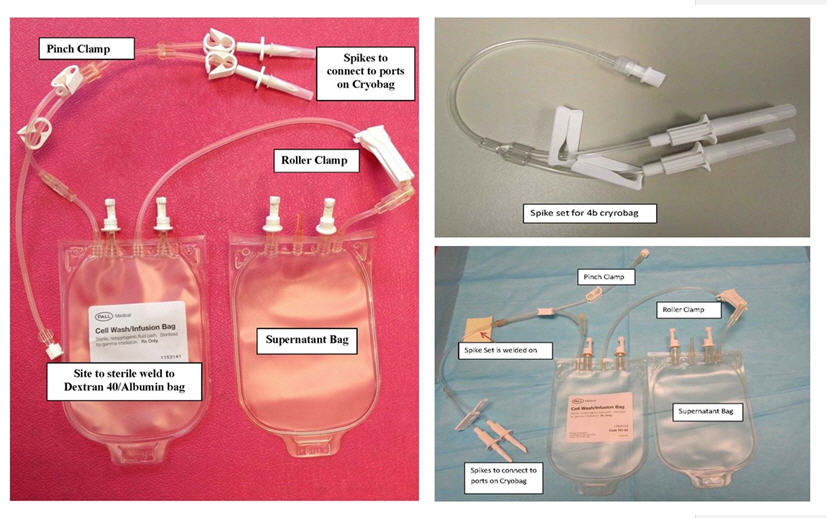
Figure 1 –Cell Wash/Infusion Set (left), Spike Set for 4b Bag Only (top right) and Cell Wash/Infusion Set for 4b bag (bottom right)
- b.
- Sterile weld the cell wash/infusion set to the Dextran 40/Albumin bag (see configuration in Figure 2).
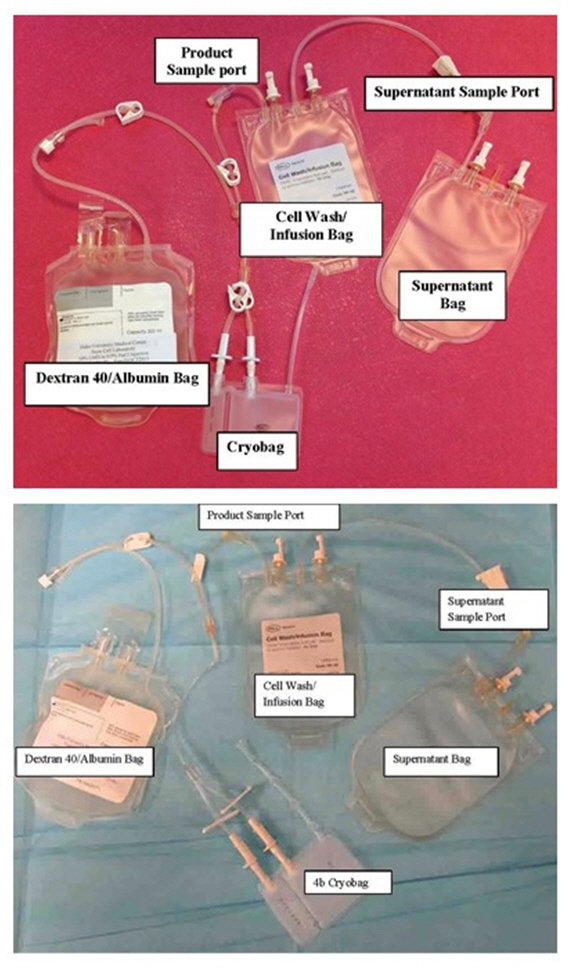
Figure 2 – Bag Configuration (top with Pall bag and bottom with 4b bag)
- c.
- For units in the Biosafe 4b bag, heat seal twice and remove the spikes to the cryobag on the Pall cell/wash kit. Sterile weld the spike couplers for the 4b kit.
- d.
- Place the infusion bag on the scale.
- e.
- Tare scale and transfer 125 grams (125 mL) of thawing solution from the Dextran 40/Albumin bag into the infusion bag (see Figure 3).

Figure 3
- f.
- Clamp off tubing between the Dextran 40/Albumin bag and the infusion bag, using pinch clamp provided with the set.
- g.
- Wrap the infusion bag with an ice mat.
- h.
- Place the cell/wash infusion set joined to the Dextran 40/Albumin bag containing thawing solution inside the hood (see Figure 4).
- i.
- Elevate and hang the Dextran 40/Albumin bag to facilitate flow (see Figure 4).
- 4
-
Assemble Materials and Equipment in Hood

Figure 4
- a.
- Assemble all materials needed in the biological safety cabinet before thawing DUCORD (see Figure 4).
- b.
- Leave one ice mat ready for the cryobag after thawing (see Figure 4).
- c.
- Assemble site specific forms and apply barcode labels to forms.
- d.
- Prepare and label all sample tubes for quality control (QC) and sterility testing with patient information, unit number, date and product type.
- e.
- Place supplies needed for the procedure inside the biological safety cabinet:
- tube rack
- 2-mL cryogenic vials
- test tubes (5 mL polystyrene tubes, 5 mL sterile culture tubes with snap cap)
- syringes
- iodine and alcohol swabs
- sterile gauze pads
- disinfected scissors
- sampling site coupler
- ice mats
- vortex
- III
-
DUCORD THAWING
- 1
-
Preparation for Thawing
- a.
- Work in the vapor phase of the LN2 freezer.
- b.
- Remove DUCORD from metal cassette.
- c.
- Confirm the ISBT barcodes on DUCORD cryobag with a second technologist or designee.
- Barcodes on DUCORD cryobag should match the labels on the metal cassette, the tie tag and on the accompanying paperwork.
- Document label check by both individuals.
- d.
- Remove overwrap plastic sealant (see Figure 5a).
- e.
- Visually inspect the cryobag for damage or cracks.
- f.
- Cut the cryobag segments, if present (see Figure 5b).
- g.
- Place the segments in a labeled 2 mL cryogenic vial (see Figure 5c) and return to LN2 freezer. Segments may be stored in the liquid or vapor phase of liquid nitrogen as long as the temperature is maintained at less than -150°C.
- 2
-
Thawing Procedure
- a.
- Gently wipe the outside surface of the cryobag with alcohol.
- b.
- Place the cryobag inside a 7 × 8 inch sterile zip-lock bag, let out the air, and seal the zip-lock bag.
- c.
- Confirm and document, with a second technologist or designee, that the label on DUCORD cells to be transplanted matches the label on the metal cassette, the tie tag and accompanying paperwork.
- d.
- Thaw the unit in a 37°C water bath until the product reaches a slushy/liquid consistency. This generally takes about 1-2 minutes.
- e.
- Treat the thawed cell suspension very gently. The cell membranes are fragile and the cells are lysed easily.
- f.
- Dry the outside of the ziplock bag containing the slushy cryobag and wipe with alcohol before placing in the biological safety cabinet.
- IV
-
DUCORD DILUTION
- 1
-
Preparation for Dilution
- a.
- Remove the thawed cryobag from zip-lock bag.
- b.
- Clean outside of the port covers with iodine swab sticks.
- c.
- Remove both port covers with alcohol cleaned scissors (Figure 6a and 6b).
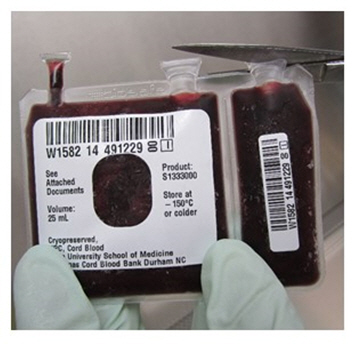
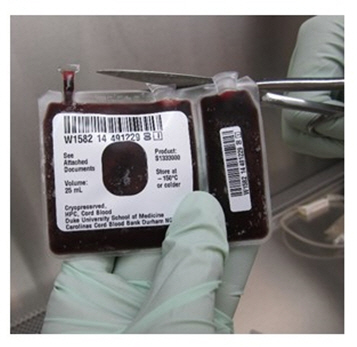
Figure 6a Figure 6b - d.
- Clean cut surfaces, first with iodine, and then with alcohol (see Figure 6c).
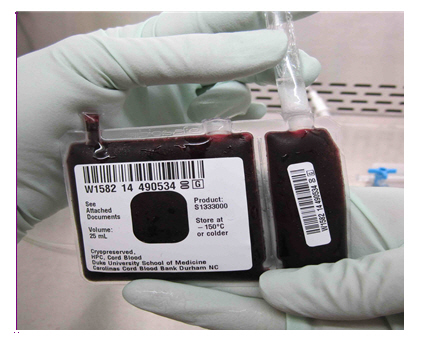
Figure 6c
- e.
- Dry the cut surfaces with sterile gauze.
- f.
- Insert the spikes (see Figure 6d) on the infusion bag (see Figure 1) in the dry and disinfected ports on the cryobag one at the time (see Figure 2).
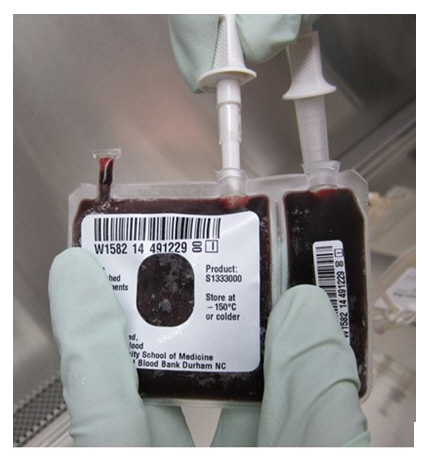
Figure 6d
- g.
- Wrap the cryobag with an ice mat.
- h.
- Unclamp tubing between infusion bag and cryobag.
- 2
-
Dilution of DUCORD
- a.
-
Slowly transfer over approximately 1-2 minutes 25 mL of chilled (4-8°C) thawing solution from the infusion bag into the cryobag (see Figure 7a) until both compartments of the cryobag bulge (see Figure 7b).
NOTE: It is important to add the thawing solution slowly over 1-2 minutes so that the dimethyl sulfoxide (DMSO) in the product is gradually diluted.
Figure 7a

Figure 7b
- b.
- Gently mix by hand the incoming thawing solution and slushy product during transfer.
- c.
- Gently rock the cryobag by hand for 1-3 minutes for complete homogenization of its contents.
- d.
- Elevate the cryobag to gradually transfer the diluted cell suspension from the cryobag into the infusion bag which is wrapped in an ice mat to keep the product cool at 4-8°C.
NOTE: At this point in the procedure, the infusion bag contains 50 mL of material from the cryobag (25 mL product + 25 mL thawing solution) mixed with the remaining 100 mL of thawing solution in the infusion bag for a total of approximately 150 mL.
- e.
- Mix the fluids during transfer by moving the bags up and down (see Figure 8).
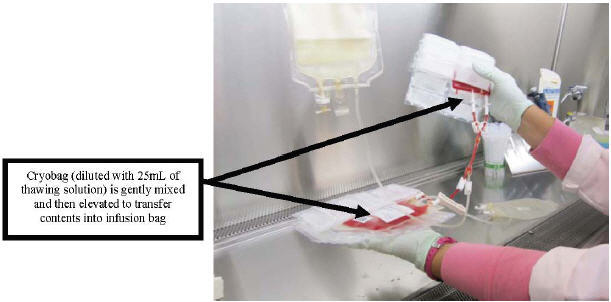
Figure 8
- f.
- Leave the remaining residual fluid and cells in the cryobag at this time.
- g.
- Gently rock the infusion bag by hand for 1-2 minutes to allow complete mixing.
- h.
- Clamp the lines between the cryobag and the infusion bag in preparation for the rinsing process.
- 3
-
First Rinse of the Cryobag
- a.
- Unclamp tubing between the Dextran 40/Albumin bag and the cryobag.
- b.
- Add approximately 25 mL of thawing solution from the Dextran 40/Albumin bag to both compartments of the cryobag.
NOTE: A total of 150 mL of thawing solution has been used to mix with the product at the completion of this step.
- c.
- Clamp tubing between the Dextran 40/Albumin bag and the cryobag after transfer.
- d.
- Apply gentle pressure and massage the cryobag to dislodge all remaining cells.
- e.
- Swirl the thawing solution around the inside of the cryobag to resuspend and harvest all remaining cells.
- f.
- Open clamp between the cryobag and the infusion bag.
- g.
- Elevate the cryobag to allow the thawing solution and cells to flow into the infusion bag.
- h.
- Gently mix the fluids during transfer by rocking the infusion bag by hand.
- i.
- Compress and roll the cryobag to remove all the remaining cell suspension.
- j.
- Drain all the solution from the cryobag into the infusion bag.
- k.
- Clamp off the line from the infusion bag in preparation for the second rinse of the cryobag.
- 4
-
Second Rinse of the Cryobag
- a.
- Again, unclamp the tubing between the Dextran 40/Albumin bag and the empty cryobag.
- b.
- Add approximately 25 mL of thawing solution from the Dextran 40/Albumin bag to both compartments of the empty cryobag.
NOTE: A total of 175 mL of thawing solution has been used to mix with the product at the completion of this step.
- c.
- Repeat steps c to j of the First Rinse of the Cryobag procedure above.
- d.
- Drain any remaining thawing solution from the Dextran 40/Albumin bag into the infusion bag.
NOTE: At this point in the procedure, the product and thawing solution are mixed together in the infusion bag for a total volume of approximately 200-205 mL.
- e.
- Heat seal tubing between the infusion bag and the cryobag, then detach the cryobag by cutting at the sealed point.
- f.
- Remove the unit identifying label from the cryobag, place it on the paperwork and discard the empty cryobag.
- g.
- Heat seal tubing between the infusion bag and the Dextran 40/Albumin bag, then detach the infusion bag by cutting at the sealed point.
NOTES: The volume of the diluted product should be close to 200 mL. A volume higher than 225 mL may cause bag breakage.
- h.
- If the product is going to be administered in the diluted state (e.g. without further washing):
- Label the infusion bag with patient and donor information per institutional standard operation procedures (Demand 128 or equivalent).
- Attach a tie tag containing the recipient and donor demographic information to the infusion bag.
- Take a 0.9 mL sample for quality control testing (see section VII).
- Transport diluted product to the site of administration to the patient in a validated transport container at 4-8°C.
- Infuse within one hour of thawing.
- Infuse the product through a 100 mL burette hemoset 170-260 micron filter.
Do not use leukoreduction filters.
- V
-
DUCORD WASH
- 1
-
Centrifugation of Thawed/Diluted Product
- a.
- Check that the centrifuge is set at 2-8°C.
- b.
- At this point, the infusion bag remains attached, by tubing, to the supernatant bag.
- c.
- Place the infusion bag inside a sterile zip-lock bag.
- d.
- Place the infusion bag in a specially designed centrifuge bucket insert (see Figure 9).
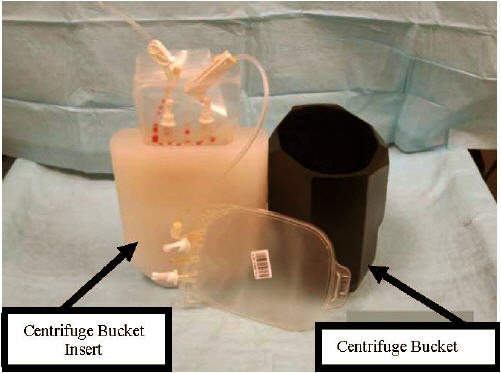
Figure 9
- e.
- Arrange the insert and the supernatant bag inside the centrifuge bucket with the supernatant bag placed between the insert and the inner wall of the centrifuge bucket (see Figure 10).
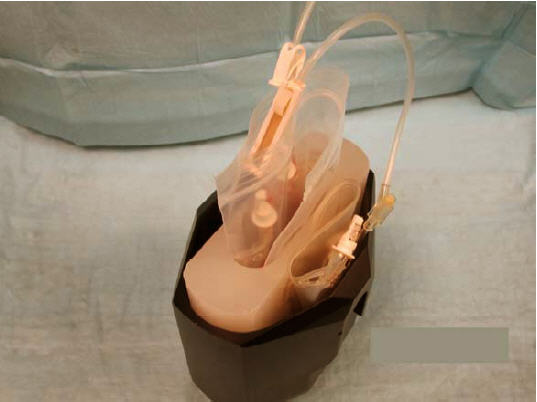
Figure 10
- f.
- Check that all clamps are closed.
- g.
- Tape the tubing so that it remains inside the centrifuge bucket during centrifugation (see Figure 11).

Figure 11
- h.
- Balance the centrifuge buckets.
- i.
- Pellet the cells via centrifugation at 880 G for 20 minutes at 2-8°C.
NOTE: In centrifuges with rotor radius equal to 25 cm, a speed of 1800 RPM is equivalent to 880 G.
- 2
-
Removal of the Supernatant
- a.
- Remove the infusion bag attached to the supernatant bag, from the centrifuge bucket.
- b.
- Place the centrifuged infusion bag in the plasma expressor and the attached supernatant bag inside a Plexiglass or equivalent tray on the scale (see Figure 12).

Figure 12
- c.
- Tare the scale.
- d.
- Open the tubing clamp between the two bags.
- e.
- Transfer most of the supernatant to the supernatant bag after leaving a volume of product appropriate for the patient's weight as determined by the transplant team (see Section II.1.i), taking into account the volume of product that will be recovered in step 3.i below.
- f.
- Be sure that the cell pellet is not disrupted during transfer of the supernatant.
- g.
- Empty the tubing between the bags by transferring the air from the supernatant bag to the infusion bag.
- h.
- Clamp the tubing to the infusion bag and remove the supernatant bag from the scale.
- i.
- Place the infusion bag on the scale.
- j.
- Record the displayed weight.
- k.
- Heat seal the tubing connecting the infusion bag and the supernatant bag, then detach the supernatant bag.
NOTE: Make sure roller clamp remains attached to the supernatant bag (see Figure 1) as this will be needed for step 3.e of the Recovery of Remaining Cells procedure.
- l.
- Gently mix the cellular contents of the infusion bag. Keep the infusion bag in the ice mat when completing subsequent steps.
NOTE: The cell suspension after the completion of this step will be referred to as cell suspension #1.
- m.
- Remove a 0.2 mL sample of cell suspension #1 for cell count and cell viability tests using sterile technique in the biological safety cabinet. Vortex the sample at the lowest speed for 3-5 seconds.
- n.
- Perform the cell count and cell viability.
- 3
-
Recovery of Remaining Cells
-
NOTE: Follow specific internal protocol of individual transplant center if the transplant center wants to refreeze the remaining cells.
- a.
- Place the supernatant bag inside a sterile zip-lock bag and then inside the centrifuge insert.
- b.
- Place the centrifuge insert and the bags in the centrifuge bucket.
- c.
- Balance the buckets.
- d.
- Pellet the cells at 880 G for 20 minutes at 2-8°C.
- e.
- Close roller clamp on supernatant bag before sterile welding.
- f.
- Sterile weld the supernatant bag to a 150 or 300 mL transfer bag.
- g.
- Place the supernatant bag containing the pelleted cells which is now attached to the transfer bag on the plasma expressor.
- h.
- Open the roller clamp between the supernatant bag and the transfer bag.
- i.
- Express the supernatant from the supernatant bag into the transfer bag without disrupting the pellet, leaving a volume appropriate to add to the infusion bag that will not to exceed the final volume requested by transplant team (See Section II.1.i and step 2.e above).
- j.
- Gently mix cellular content of the supernatant bag after expression of supernatant and then draw the contents into a 20 or 30 mL syringe using the supernatant sample port (see Figure 2).
NOTE: The cell suspension after the completion of this step will be referred to as cell suspension #2.
- k.
- Measure the volume in the syringe.
- l.
- Transfer 0.2 mL sample of cell suspension #2 from the syringe into a 5 mL test tube for a cell count using sterile technique in the biological safety cabinet. Vortex the sample at the lowest speed for 3-5 seconds.
- m.
- Perform the cell count.
- n.
- Inject cell suspension #2 into the product sample port (in Figure 2) on the tubing attached to the infusion bag containing cell suspension #1 to combine the two cell suspensions.
- o.
- Determine the combined volume and/or weight of the final product.
- p.
- Proceed to the next section for transplantation preparation steps.
- VI
-
PREPARATION OF DUCORD FOR TRANSPLANTATION
- 1
-
BAG Method for Adult Recipients
- a.
- Remove a 0.9 mL sample for QC testing from the product sample port (see Figure 2) on the tubing attached to the infusion bag using sterile technique with a sterile needle and syringe in a biological safety cabinet. Vortex sample at the lowest speed for 3-5 seconds. This QC sample is used to perform viability and a total nucleated cell count and other testing as recommend (see section VII of the procedure for QUALITY CONTROL TESTS).
- b.
- Calculate viable cell recovery using the following formula:
Total Viable Nucleated Cells on the Infusion Ready Product
Total Viable Nucleated Cells of Cryopreserved Unit Provided on Feedback Sheet - c.
- Record all values on the designated laboratory thawing form.
- d.
- Label the infusion bag with patient and donor information per institutional standard operation procedures (e.g. Demand 128 or equivalent).
- e.
- Attach a tie tag containing the recipient and donor demographic information to the infusion bag.
- f.
- Prepare a saline wash bag per physician order by transferring 20-100 mL of 0.9% Sodium Chloride solution into a 150 mL transfer bag with extension tubing. This saline solution will be used to wash the infusion bag after the product is infused into the patient.
- g.
- Sterile weld the infusion bag to the saline wash bag (see Figure 13). Clamp the tubing between the infusion bag and the saline wash bag, using the clamp on the tubing connected to the infusion bag, on the side closer to the infusion bag.
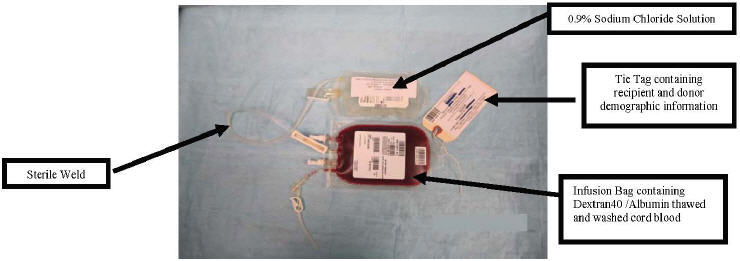
Figure 13
- h.
- Complete appropriate laboratory forms to document the thawing process and final volumes.
- i.
- Confirm and document, with a second technologist or designee, the ISBT or equivalent barcode affixed and tie tag attached on bag of cells to be transplanted.
- j.
- Transport product to the site of administration to the patient in a validated transport container at 4-8°C.
- k.
- Infuse within four hours of thawing.
- l.
- Infuse the product through a 100 mL burette hemoset 170-260 micron filter at the bedside. Do not use leukoreduction filters
.
- 2
-
SYRINGE Method for Pediatric Recipients or Recipients on Fluid Restrictions
- a.
- Place an ISBT barcode label on a 60 mL and 3 mL syringe.
- b.
- Remove 100 mL burette hemoset 170-260 micron filter set from its packaging.
- c.
- Clamp the roller clamp on the hemoset tubing.
- d.
- Spike the 100 mL burette hemoset 170-260 micron filter set into the port on the infusion bag.
- e.
- Attach a 3-way stopcock to the end of the 100 mL burette hemoset filter line. The stopcock will accommodate a 3 mL syringe (for QC tests) and 60 mL syringe (see Figure 14).
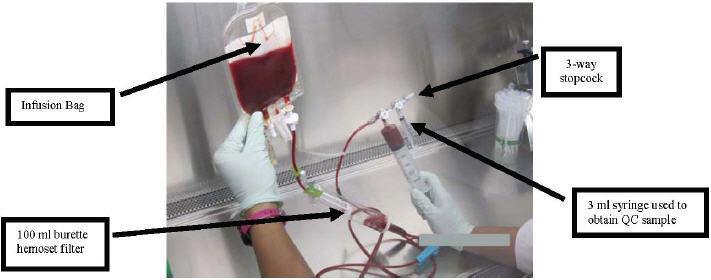
Figure 14
- f.
- Mix and pull the cellular product into the 60 mL syringe so the entire volume can be accurately measured.
- g.
- Attach the 3 mL syringe to the open port on the stopcock.
- h.
- Adjust the stopcock and use the 3 mL syringe to remove a 0.9 mL sample of product to be used for QC testing. This QC sample is used to perform viability and a total nucleated cell count. Refer to section VII of the procedure for QUALITY CONTROL TESTS.
- i.
- Detach the 60 mL syringe containing the cellular product and attach it to the lipid compatible 3-way stopcock clamp, the neutral displacement needleless, connector, and the 60 inch, 2.4 mL extension set tubing (see Figure 15).
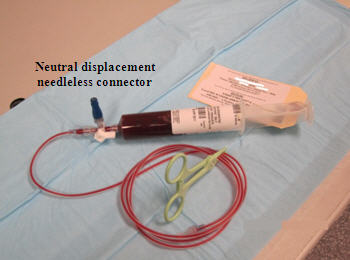
Figure 15
- j.
- Prime the extension set tubing with cellular product (2.4 mL capacity). Be sure to place the stopcock in the OFF position after the tubing has been primed.
- k.
- Attach rubber shod clamp to the tubing to ensure there is no leakage.
- l.
- Calculate viable cell recovery using the following formula:
Total Viable Nucleated Cells on the Infusion Ready Product
Total Viable Nucleated Cells of Cryopreserved Unit Provided on Feedback Sheet - m.
- Record all values on the laboratory thawing form.
- n.
- Label the syringe with patient and donor information per institutional standard operation procedures (Demand 128 or equivalent).
- o.
- Attach a tie tag containing the recipient and donor demographic information to the syringe.
- p.
- Complete appropriate laboratory paperwork to record all steps in the procedure and volumes recovered.
- q.
- Confirm with a second technologist or designee that the affixed label on the syringe and tie tag attached to the syringe barrel match. Document that this step was completed on transplant center form.
- r.
- Transport product to the site of administration in a validated transport container at 4-8°C.
- s.
- Infuse within four hours of thawing.
- t.
-
Do not use leukoreduction filters.
- VII
-
QUALITY CONTROL TESTS
- Cell Counts: perform on final product to be infused, diluted product (optional), cell suspension #1, cell suspension #2, combined cell suspension ( #1 and #2) and cells for freezing from the supernatant bag if requested by the transplant center.
- Viability test by Trypan Blue: perform on initial cell product after first centrifugation and on the final product.
- Colony assay for CFU-GM, GEMM and BFU-E: perform on final product to be infused.
- Viable CD34+ determination by flow analysis: perform on final product to be infused.
- Sterility Test: perform on 10 mL of supernatant from the final wash (5 mL for aerobic and 5 mL for anaerobic cultures).
- RFLP or equivalent DNA-based identity test: perform on approximately 1 × 106 cells (0.2 mL) from infusion-ready product, upon request.
- VIII
-
EMERGENCY PRODUCT RECOVERY IN THE EVENT OF A CONTAINER FAILURE
- Handle the cryobag with extreme caution when removing it from the liquid nitrogen, metal cassette and the protective overwrap. Cryobags can be very fragile. This is applicable both during inspection of the product upon arrival and during the thawing process.
- Wipe the external surface of the cryobag with isopropyl alcohol before the cryobag is placed inside a sterile zip-lock bag. This process positions the thawing laboratory to 30 of 30 potentially recover the product in the case of an unexpected leak or container failure during thawing or centrifugation.
- Contact the CCBB Laboratory Supervisor at 919-257-9273 or 919-668-2066 if, during thawing, any portion of the product or container appears to be damaged or compromised. The CCBB will provide advice to determine the best way to recovery the product from the compromised container. The CCBB will also provide further instruction for return of the container, if requested, and a prompt investigation will follow. The transplant physician and the local laboratory director should also be notified immediately.
- If the bag is compromised, move further handling into biological safety cabinet after thawing.
- It is the transplant physician's (or designee's) responsibility to determine whether the product will be used or discarded if the container is compromised at any step of the procedure.
- If the transplant physician (or designee) decides that the product should be used, recovery of the product may be attempted as described below.
- a.
- Use a long (3-5 inch) blunt needle (a sterile spinal needle with trochar removed if available).
- b.
- Attach the blunt needle to a sterile 60 mL syringe.
- c.
- Aspirate the product and inject into a sterile transfer bag.
NOTE: More than one syringe may be needed if the volume in the bag exceeds 60mL at the time of violation of the integrity of the bag.
- d.
- Complete processing of the transferred product as indicated.
- e.
- Perform sterility test on the product sample after final processing.
NOTE: Alert the clinical team at the transplant center that the product was violated and could potentially be contaminated.
- Save the ruptured bag if possible. When contacted about the container failure, the CCBB Laboratory Supervisor will provide further instructions for return of the container, if requested. A prompt CCBB investigation will follow, as applicable.
Distributed by:
Duke University School of Medicine
Carolinas Cord Blood Bank
North Pavilion Building, Suite 1400
2400 Pratt Street
Durham, NC 27705
U.S. License #1870