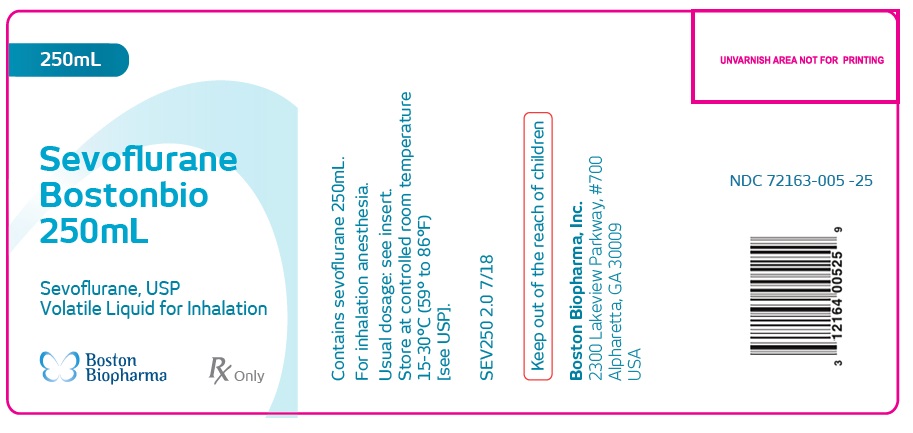
Sevoflurane
Bostonbio
250mL
Sevoflurane, USP
Volatile Liquid for Inhalation
Contains sevoflurane 250mL.
For inhalation anesthesia.
Usual dosage: see insert.
Store at controlled room temperature
15-30°C (59° to 86°F)
[see USP].
SEV250 2.0 7/18
Keep out of the reach of children
Boston Biopharma, Inc.
2300 Lakeview Parkway, #700
Alpharetta, GA 30009
USA
NDC 72163-005 -25