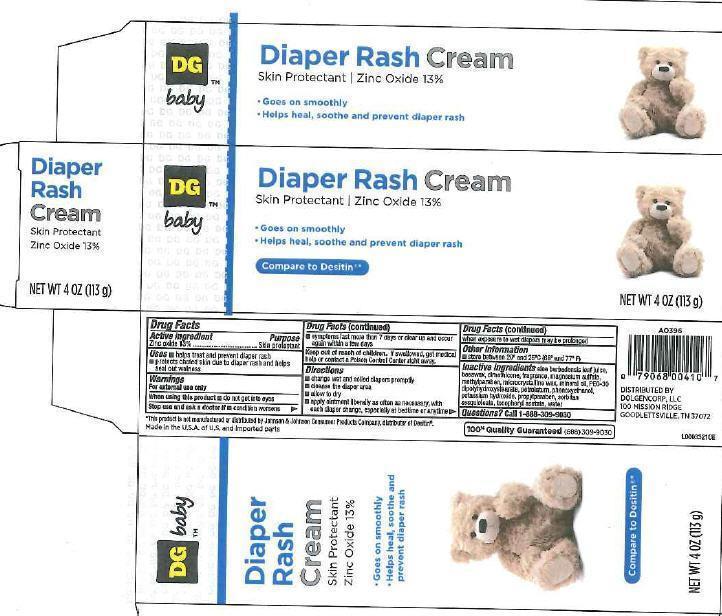
DIAPER RASH- zinc oxide cream
DolGenCorp, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingrdients
Active ingredient
Zinc oxide 13%
Purpose
Purpose
Skin protectant
Uses
Uses
- helps treat and prevent diaper rash
- protects chafed skin due to diaper rash and helps seal out wetness
Warnings
Warnings
For external use only
When using this product
When using this product
Stop use
Stop use and ask a doctor if
- condtin worsens
- symptoms last more tha 7 days or clear up and occur again within a few days
Keep out of reach of children
Keep out of reach of children. If swallowed, get edical help or contact a Poison Control Center right away
Directions
Directions
- change wet ad soiled diapers promptly
- cleanse the diaper area
- allow to dry
- apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged.
other information
Other information2.2ex0em2.2ex0emstore between 20º and 25ºC (68º and 77º F)
Inactive ingredients
Inactive ingredients aloe barbadensis leaf juice, beeswax, dimethicone, ethylhexylglycerin, fragrance, magnesium sulfate, microcrystalline wax, mineral oil, PEG-30 dipolyhydroxystearate, petrolatum, phenoxyethanol, potassium hydroxide, sorbitan sesquioleate, tocoperyl acetate, water
Questions
Questions? Call 1-888-309-9030
Disclaimer
This product is not manufactured or distributed by Johnson + Johnson Consumer Products Company, distributor of Desitin
Made in the U.S.A. of U.S. and imported parts
Adverse reactions
DISTRIBUTED BY
DOLGENCORP, LLC
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072
Principal display panel
DG baby
Diaper
Rash
Cream
Skin Protectant
Zinc Oxide 13%
- Goes on smoothly
- Helps heal, soothe and prevent diaper rash
Compare to Desitin
NET WT 4 OZ (113 g)