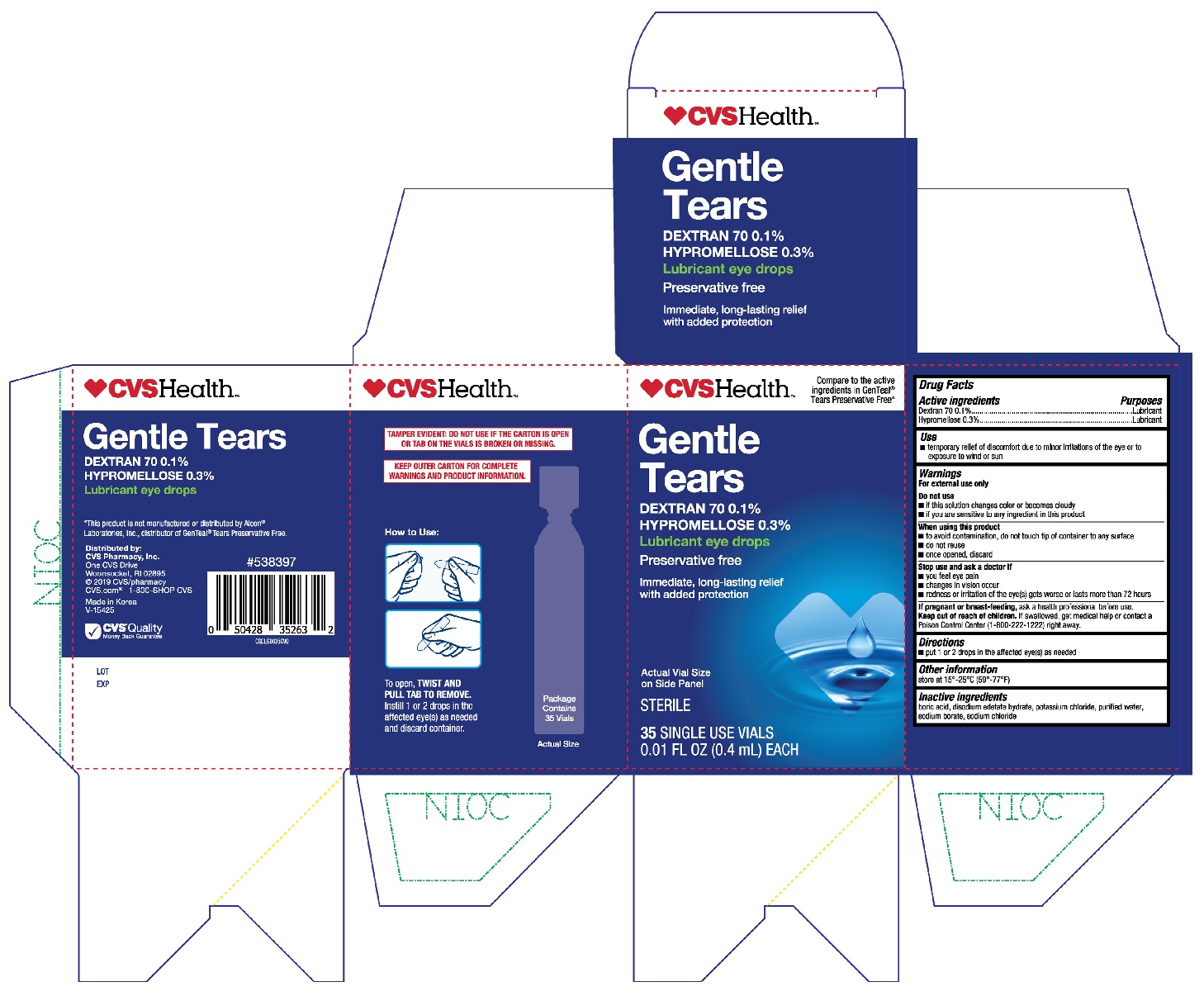
Active ingredients
Dextran 70 0.1%
Hypromellose 0.3%
Purposes
Lubricant
Lubricant
Use
- temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
Warnings
For external use only
Do not use
- if this solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse or lasts more than 72 hours
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- put 1 or 2 drops in the affected eye(s) as needed
Other information
store at 15°-25°C (59°-77°F)
Inactive ingredients
boric acid, disodium edetate hydrate, potassium chloride, purified water, sodium borate, sodium chloride
CVS Gentle Tears 35ct
CVS Pharmacy, Inc.
