TOBRAMYCIN- tobramycin solution
Sandoz Inc
----------
Tobramycin Inhalation Solution, USP
Nebulizer Solution – For Inhalation Use Only
DESCRIPTION
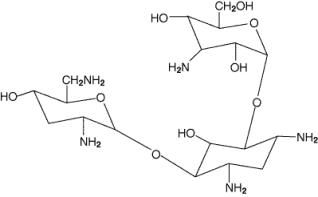
Tobramycin Inhalation Solution, USP is a solution for inhalation. It is a sterile, clear, slightly yellow, non-pyrogenic, aqueous solution with the pH and salinity adjusted specifically for administration by a compressed air driven reusable nebulizer. The chemical formula for tobramycin is C18H37N5O9 and the molecular weight is 467.52. Tobramycin is O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine. The structural formula for tobramycin is:
Each single-use 5 mL ampule contains 300 mg tobramycin and 11.25 mg sodium chloride in sterile water for injection. Sulfuric acid and sodium hydroxide are added to adjust the pH to 6.0. Nitrogen is used for sparging. All ingredients meet USP requirements. The formulation contains no preservatives.
CLINICAL PHARMACOLOGY
Tobramycin inhalation solution is specifically formulated for administration by inhalation. When inhaled, tobramycin is concentrated in the airways.
Pharmacokinetics
Tobramycin inhalation solution contains tobramycin, a cationic polar molecule that does not readily cross epithelial membranes.(1) The bioavailability of tobramycin inhalation solution may vary because of individual differences in nebulizer performance and airway pathology.(2) Following administration of tobramycin inhalation solution, tobramycin remains concentrated primarily in the airways.
Sputum Concentrations: Ten minutes after inhalation of the first 300-mg dose of tobramycin inhalation solution, the average concentration of tobramycin was 1237 mcg/g (ranging from 35 to 7417 mcg/g) in sputum. Tobramycin does not accumulate in sputum; after 20 weeks of therapy with the tobramycin inhalation solution regimen, the average concentration of tobramycin at ten minutes after inhalation was 1154 mcg/g (ranging from 39 to 8085 mcg/g) in sputum. High variability of tobramycin concentration in sputum was observed. Two hours after inhalation, sputum concentrations declined to approximately 14% of tobramycin levels at ten minutes after inhalation.
Serum Concentrations: The average serum concentration of tobramycin one hour after inhalation of a single 300-mg dose of tobramycin inhalation solution by cystic fibrosis patients was 0.95 mcg/mL. After 20 weeks of therapy on the tobramycin inhalation solution regimen, the average serum tobramycin concentration one hour after dosing was 1.05 mcg/mL.
Elimination: The elimination half-life of tobramycin from serum is approximately 2 hours after intravenous (IV) administration. Assuming tobramycin absorbed following inhalation behaves similarly to tobramycin following IV administration, systemically absorbed tobramycin is eliminated principally by glomerular filtration. Unabsorbed tobramycin, following tobramycin inhalation solution administration, is probably eliminated primarily in expectorated sputum.
Microbiology
Tobramycin is an aminoglycoside antibiotic produced by Streptomyces tenebrarius.(1) It acts primarily by disrupting protein synthesis, leading to altered cell membrane permeability, progressive disruption of the cell envelope, and eventual cell death.(3)
Tobramycin has in vitroactivity against a wide range of gram-negative organisms including Pseudomonas aeruginosa. It is bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Susceptibility Testing
A single sputum sample from a cystic fibrosis patient may contain multiple morphotypes of Pseudomonas aeruginosa and each morphotype may have a different level of in vitrosusceptibility to tobramycin. Treatment for 6 months with tobramycin inhalation solution in two clinical studies did not affect the susceptibility of the majority of P. aeruginosa isolates tested; however, increased minimum inhibitory concentrations (MICs) were noted in some patients. The clinical significance of this information has not been clearly established in the treatment of P. aeruginosa in cystic fibrosis patients. For additional information regarding the effects of tobramycin inhalation solution on P. aeruginosa MIC values and bacterial sputum density, please refer to the CLINICAL STUDIES section.
The in vitroantimicrobial susceptibility test methods used for parenteral tobramycin therapy can be used to monitor the susceptibility of P. aeruginosa isolated from cystic fibrosis patients. If decreased susceptibility is noted, the results should be reported to the clinician.
Susceptibility breakpoints established for parenteral administration of tobramycin do not apply to aerosolized administration of tobramycin inhalation solution. The relationship between in vitrosusceptibility test results and clinical outcome with tobramycin inhalation solution therapy is not clear.
INDICATIONS AND USAGE
Tobramycin inhalation solution is indicated for the management of cystic fibrosis patients with P. aeruginosa.
Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV1) <25% or >75% predicted, or patients colonized with Burkholderia cepacia (see CLINICAL STUDIES).
CONTRAINDICATIONS
Tobramycin inhalation solution is contraindicated in patients with a known hypersensitivity to any aminoglycoside.
WARNINGS
Caution should be exercised when prescribing tobramycin inhalation solution to patients with known or suspected renal, auditory, vestibular, or neuromuscular dysfunction. Patients receiving concomitant parenteral aminoglycoside therapy should be monitored as clinically appropriate.
Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides cross the placenta, and streptomycin has been associated with several reports of total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. Patients who use tobramycin inhalation solution during pregnancy, or become pregnant while taking tobramycin inhalation solution should be apprised of the potential hazard to the fetus.
Ototoxicity
Ototoxicity, as measured by complaints of hearing loss or by audiometric evaluations, did not occur with tobramycin inhalation solution therapy during clinical studies. However, transient tinnitus occurred in eight tobramycin inhalation solution-treated patients versus no placebo patients in the clinical studies. Tinnitus may be a sentinel symptom of ototoxicity, and therefore the onset of this symptom warrants caution (see ADVERSE REACTIONS). Ototoxicity, manifested as both auditory and vestibular toxicity, has been reported with parenteral aminoglycosides. Vestibular toxicity may be manifested by vertigo, ataxia or dizziness.
In postmarketing experience, patients receiving tobramycin inhalation solution have reported hearing loss. Some of these reports occurred in patients with previous or concomitant treatment with systemic aminoglycosides. Patients with hearing loss frequently reported tinnitus.
Nephrotoxicity
Nephrotoxicity was not seen during tobramycin inhalation solution clinical studies but has been associated with aminoglycosides as a class. If nephrotoxicity occurs in a patient receiving tobramycin inhalation solution, tobramycin therapy should be discontinued until serum concentrations fall below 2 mcg/mL.
Muscular Disorders
Tobramycin inhalation solution should be used cautiously in patients with neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, since aminoglycosides may aggravate muscle weakness because of a potential curare-like effect on neuromuscular function.
Bronchospasm
Bronchospasm can occur with inhalation of tobramycin inhalation solution. In clinical studies of tobramycin inhalation solution, changes in FEV1 measured after the inhaled dose were similar in the tobramycin inhalation solution and placebo groups. Bronchospasm should be treated as medically appropriate.
PRECAUTIONS
Information for Patients
NOTE: In addition to information provided below, a Patient Medication Guide providing instructions for proper use of tobramycin inhalation solution is contained inside the package.
Safety Information
Tobramycin inhalation solution is in a class of antibiotics that have caused hearing loss, dizziness, kidney damage, and harm to a fetus. Ringing in the ears and hoarseness were two symptoms that were seen in more patients taking tobramycin inhalation solution than placebo in research studies. Patients with cystic fibrosis can have many symptoms. Some of these symptoms may be related to your medications. If you have new or worsening symptoms, you should tell your doctor.
Hearing: You should tell your doctor if you have ringing in the ears, dizziness, or any changes in hearing.
Kidney Damage: Inform your doctor if you have any history of kidney problems.
Pregnancy: If you want to become pregnant or are pregnant while on tobramycin inhalation solution, you should talk with your doctor about the possibility of tobramycin inhalation solution causing any harm.
Nursing Mothers: If you are nursing a baby, you should talk with your doctor before using tobramycin inhalation solution.
Tobramycin Inhalation Solution, USP Packaging
Tobramycin inhalation solution comes in a single dose, ready-to-use ampule containing 300 mg tobramycin. Each foil pouch contains 4 ampules, for 2 days of tobramycin inhalation solution therapy.
Dosage
The 300 mg dose of tobramycin inhalation solution is the same for patients regardless of age or weight. Tobramycin inhalation solution has not been studied in patients less than 6 years old. Doses should be inhaled as close to 12 hours apart as possible and not less than 6 hours apart.
You should not mix tobramycin inhalation solution with dornase alfa (PULMOZYME®, Genentech) in the nebulizer.
If you are taking several medications the recommended order is as follows: bronchodilator first, followed by chest physiotherapy, then other inhaled medications and, finally, tobramycin inhalation solution.
Treatment Schedule
You should take tobramycin inhalation solution in repeated cycles of 28 days on drug followed by 28 days off drug. You should take tobramycin inhalation solution twice a day during the 28-day period on drug.
How To Administer Tobramycin Inhalation Solution, USP
THIS INFORMATION IS NOT INTENDED TO REPLACE CONSULTATION WITH YOUR PHYSICIAN AND CF CARE TEAM ABOUT PROPERLY TAKING MEDICATION OR USING INHALATION EQUIPMENT.
Tobramycin inhalation solution is specifically formulated for inhalation using a PARI LC PLUS™ Reusable Nebulizer and a DeVilbiss® Pulmo-Aide® air compressor. Tobramycin inhalation solution can be taken at home, school, or at work. The following are instructions on how to use the DeVilbiss Pulmo-Aide air compressor and PARI LC PLUS Reusable Nebulizer to administer tobramycin inhalation solution.
You will need the following supplies:
- •
- Tobramycin Inhalation Solution, USP plastic ampule (vial)
- •
- DeVilbiss Pulmo-Aide air compressor
- •
- PARI LC PLUS Reusable Nebulizer
- •
- Tubing to connect the nebulizer and compressor
- •
- Clean paper or cloth towels
- •
- Nose clips (optional)
It is important that your nebulizer and compressor function properly before starting your tobramycin inhalation solution therapy.
Note: Please refer to the manufacturers’ care and use instructions for important information.
Preparing Your Tobramycin Inhalation Solution, USP for Inhalation
1. Wash your hands thoroughly with soap and water.
2a. Tobramycin Inhalation Solution, USP is packaged with 4 ampules per foil pouch.
2b. Separate one ampule by gently pulling apart at the bottom tabs. Store all remaining ampules in the refrigerator as directed.
3. Lay out the contents of a PARI LC PLUS Reusable Nebulizer package on a clean, dry paper or cloth towel. You should have the following parts:
- •
- Nebulizer Top and Bottom (Nebulizer Cup) Assembly
- •
- Inspiratory Valve Cap
- •
- Mouthpiece with Valve
- •
- Tubing
4. Remove the Nebulizer Top from the Nebulizer Cup by twisting the Nebulizer Top counter-clockwise, and then lifting off. Place the Nebulizer Top on the clean paper or cloth towel. Stand the Nebulizer Cup upright on the towel.
5. Connect one end of the tubing to the compressor air outlet. The tubing should fit snugly. Plug in your compressor to an electrical outlet.
6. Open the Tobramycin Inhalation Solution, USP ampule by holding the bottom tab with one hand and twisting off the top of the ampule with the other hand. Be careful not to squeeze the ampule until you are ready to empty its contents into the Nebulizer Cup.
7. Squeeze all the contents of the ampule into the Nebulizer Cup.
8. Replace the Nebulizer Top. Note: In order to insert the Nebulizer Top into the Nebulizer Cup, the semi-circle halfway down the stem of the Nebulizer Top should face the Nebulizer Outlet.
9. Attach the Mouthpiece to the Nebulizer Outlet. Then firmly push the Inspiratory Valve Cap in place on the Nebulizer Top. Note: the Inspiratory Valve Cap will fit snugly.
10. Connect the free end of the tubing from the compressor to the Air Intake on the bottom of the nebulizer, making sure to keep the nebulizer upright. Press the tubing on the Air Intake firmly.
Tobramycin Inhalation Solution, USP Treatment
1. Turn on the compressor.
2. Check for a steady mist from the Mouthpiece. If there is no mist, check all tubing connections and confirm that the compressor is working properly.
3. Sit or stand in an upright position that will allow you to breathe normally.
4. Place Mouthpiece between your teeth and on top of your tongue and breathe normally only through your mouth. Nose clips may help you breathe through your mouth and not through your nose. Do not block airflow with your tongue.
5. Continue treatment until all your tobramycin inhalation solution is gone, and there is no longer any mist being produced. You may hear a sputtering sound when the Nebulizer Cup is empty. The entire tobramycin inhalation solution treatment should take approximately 15 minutes to complete. Note: if you are interrupted, need to cough or rest during your tobramycin inhalation solution treatment, turn off the compressor to save your medication. Turn the compressor back on when you are ready to resume your therapy.
6. Follow the nebulizer cleaning and disinfecting instructions after completing therapy.
Cleaning Your Nebulizer
To reduce the risk of infection, illness or injury from contamination, you must thoroughly clean all parts of the nebulizer as instructed after each treatment. Never use a nebulizer with a clogged nozzle. If the nozzle is clogged, no aerosol mist is produced, which will alter the effectiveness of the treatment. Replace the nebulizer if clogging occurs.
1. Remove tubing from nebulizer and disassemble nebulizer parts.
2. Wash all parts (except tubing) with warm water and liquid dish soap.
3. Rinse thoroughly with warm water and shake out water.
4. Air dry or hand dry nebulizer parts on a clean, lint-free cloth. Reassemble nebulizer when dry, and store.
5. You can also wash all parts of the nebulizer in a dishwasher (except tubing). Place the nebulizer parts in a dishwasher basket, then place on the top rack of the dishwasher. Remove and dry the parts when the cycle is complete.
Disinfecting Your Nebulizer
Your nebulizer is for your use only - Do not share your nebulizer with other people. You must regularly disinfect the nebulizer. Failure to do so could lead to serious or fatal illness.
Clean the nebulizer as described above. Every other treatment day, disinfect the nebulizer parts (except tubing) by boiling them in water for a full 10 minutes. Dry parts on a clean, lint-free cloth.
Care and Use of Your Pulmo-Aide Compressor
Follow the manufacturer’s instructions for care and use of your compressor.
Filter Change:
1. DeVilbiss Compressor filters should be changed every six months or sooner if filter turns completely gray in color.
Compressor Cleaning:
1. With power switch in the “Off” position, unplug power cord from wall outlet.
2. Wipe outside of the compressor cabinet with a clean, damp cloth every few days to keep dust free.
Caution: Do not submerge in water; doing so will result in compressor damage.
Storage Instructions
You should store tobramycin inhalation solution ampules in a refrigerator (2°C-8°C or 36°C-46°F). However, when you don’t have a refrigerator available (e.g., transporting your tobramycin inhalation solution), you may store the foil pouches (opened or unopened) at room temperature (up to 25°C/77°F) for up to 28 days.
Avoid exposing tobramycin inhalation solution ampules to intense light.
Unrefrigerated tobramycin inhalation solution, which is normally slightly yellow, may darken with age; however, the color change does not indicate any change in the quality of the product.
You should not use tobramycin inhalation solution if it is cloudy, if there are particles in the solution, or if it has been stored at room temperature for more than 28 days. You should not use tobramycin inhalation solution beyond the expiration date stamped on the ampule.
Additional Information
Nebulizer: 1-800-327-8632
Compressor: 1-800-338-1988
Tobramycin Inhalation Solution, USP: 1-800-525-8747
Laboratory Tests
Audiograms
Clinical studies of tobramycin inhalation solution did not identify hearing loss using audiometric tests which evaluated hearing up to 8000 Hz. Physicians should consider an audiogram for patients who show any evidence of auditory dysfunction, or who are at increased risk for auditory dysfunction. Tinnitus may be a sentinel symptom of ototoxicity, and therefore the onset of this symptom warrants caution.
Serum Concentrations
In patients with normal renal function treated with tobramycin inhalation solution, serum tobramycin concentrations are approximately 1 mcg/mL 1 hour after dose administration and do not require routine monitoring. Serum concentrations of tobramycin in patients with renal dysfunction or patients treated with concomitant parenteral tobramycin should be monitored at the discretion of the treating physician.
The serum concentration of tobramycin should only be monitored through venipuncture and not finger prick blood sampling. Contamination of the skin of the fingers with tobramycin may lead to falsely increased measurements of serum levels of the drug. This contamination cannot be completely avoided by hand washing before testing.
Renal Function
The clinical studies of tobramycin inhalation solution did not reveal any imbalance in the percentage of patients in the tobramycin inhalation solution and placebo groups who experienced at least a 50% rise in serum creatinine from baseline (see ADVERSE REACTIONS). Laboratory tests of urine and renal function should be conducted at the discretion of the treating physician.
Drug Interactions
In clinical studies of tobramycin inhalation solution, patients taking tobramycin inhalation solution concomitantly with dornase alfa (PULMOZYME, Genentech), ß-agonists, inhaled corticosteroids, other anti-pseudomonal antibiotics, or parenteral aminoglycosides demonstrated adverse experience profiles similar to the study population as a whole.
Concurrent and/or sequential use of tobramycin inhalation solution with other drugs with neurotoxic, nephrotoxic, or ototoxic potential should be avoided. Some diuretics can enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue. Tobramycin inhalation solution should not be administered concomitantly with ethacrynic acid, furosemide, urea, or intravenous mannitol. The interaction between inhaled mannitol and tobramycin inhalation solution has not been evaluated.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year rat inhalation toxicology study to assess carcinogenic potential of tobramycin inhalation solution has been completed. Rats were exposed to tobramycin inhalation solution for up to 1.5 hours per day for 95 weeks. The clinical formulation of the drug was used for this carcinogenicity study. Serum levels of tobramycin of up to 35 mcg/mL were measured in rats, in contrast to the average 1 mcg/mL levels observed in cystic fibrosis patients in clinical trials. There was no drug-related increase in the incidence of any variety of tumor.
Additionally, tobramycin inhalation solution has been evaluated for genotoxicity in a battery of in vitro and in vivo tests. The Ames bacterial reversion test, conducted with 5 tester strains, failed to show a significant increase in revertants with or without metabolic activation in all strains. Tobramycin was negative in the mouse lymphoma forward mutation assay, did not induce chromosomal aberrations in Chinese hamster ovary cells, and was negative in the mouse micronucleus test.
Subcutaneous administration of up to 100 mg/kg of tobramycin did not affect mating behavior or cause impairment of fertility in male or female rats.
Pregnancy
Teratogenic Effects – Pregnancy Category D
(See WARNINGS)
No reproduction toxicology studies have been conducted with tobramycin inhalation solution. However, subcutaneous administration of tobramycin at doses of 100 or 20 mg/kg/day during organogenesis was not teratogenic in rats or rabbits, respectively. Doses of tobramycin ≥40 mg/kg/day were severely maternally toxic to rabbits and precluded the evaluation of teratogenicity. Aminoglycosides can cause fetal harm (e.g., congenital deafness) when administered to a pregnant woman. Ototoxicity was not evaluated in offspring during nonclinical reproduction toxicity studies with tobramycin. If tobramycin inhalation solution is used during pregnancy, or if the patient becomes pregnant while taking tobramycin inhalation solution, the patient should be apprised of the potential hazard to the fetus.
Nursing Mothers
It is not known if tobramycin will reach sufficient concentrations after administration by inhalation to be excreted in human breast milk. Because of the potential for ototoxicity and nephrotoxicity in infants, a decision should be made whether to terminate nursing or discontinue tobramycin inhalation solution.
Pediatric Use
The safety and efficacy of tobramycin inhalation solution have not been studied in pediatric patients under 6 years of age.
Geriatric Use
Clinical studies of tobramycin inhalation solution did not include patients aged 65 years and over. Tobramycin is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function (see WARNINGS – Nephrotoxicity; PRECAUTIONS – Serum Concentrations).
ADVERSE REACTIONS
Tobramycin inhalation solution was generally well tolerated during two clinical studies in 258 cystic fibrosis patients ranging in age from 6 to 48 years. Patients received tobramycin inhalation solution in alternating periods of 28 days on and 28 days off drug in addition to their standard cystic fibrosis therapy for a total of 24 weeks.
Voice alteration and tinnitus were the only adverse experiences reported by significantly more tobramycin inhalation solution-treated patients. Thirty-three patients (13%) treated with tobramycin inhalation solution complained of voice alteration compared to 17 (7%) placebo patients. Voice alteration was more common in the on-drug periods.
Eight patients from the tobramycin inhalation solution group (3%) reported tinnitus compared to no placebo patients. All episodes were transient, resolved without discontinuation of the tobramycin inhalation solution treatment regimen, and were not associated with loss of hearing in audiograms. Tinnitus is one of the sentinel symptoms of cochlear toxicity, and patients with this symptom should be carefully monitored for high frequency hearing loss. The numbers of patients reporting vestibular adverse experiences such as dizziness were similar in the tobramycin inhalation solution and placebo groups.
Nine (3%) patients in the tobramycin inhalation solution group and nine (3%) patients in the placebo group had increases in serum creatinine of at least 50% over baseline. In all nine patients in the tobramycin inhalation solution group, creatinine decreased at the next visit.
Table 1 lists the percent of patients with treatment-emergent adverse experiences (spontaneously reported and solicited) that occurred in >5% of tobramycin inhalation solution patients during the two Phase III studies.
|
Adverse Event |
Tobramycin |
Placebo |
|
Cough Increased |
46.1 |
47.3 |
|
Pharyngitis |
38.0 |
39.3 |
|
Sputum Increased |
37.6 |
39.7 |
|
Asthenia |
35.7 |
39.3 |
|
Rhinitis |
34.5 |
33.6 |
|
Dyspnea |
33.7 |
38.5 |
|
Fever* |
32.9 |
43.5 |
|
Lung Disorder |
31.4 |
31.3 |
|
Headache |
26.7 |
32.1 |
|
Chest Pain |
26.0 |
29.8 |
|
Sputum Discoloration |
21.3 |
19.8 |
|
Hemoptysis |
19.4 |
23.7 |
|
Anorexia |
18.6 |
27.9 |
|
Lung Function Decreased† |
16.3 |
15.3 |
|
Asthma |
15.9 |
20.2 |
|
Vomiting |
14.0 |
22.1 |
|
Abdominal Pain |
12.8 |
23.7 |
|
Voice Alteration |
12.8 |
6.5 |
|
Nausea |
11.2 |
16.0 |
|
Weight Loss |
10.1 |
15.3 |
|
Pain |
8.1 |
12.6 |
|
Sinusitis |
8.1 |
9.2 |
|
Ear Pain |
7.4 |
8.8 |
|
Back Pain |
7.0 |
8.0 |
|
Epistaxis |
7.0 |
6.5 |
|
Taste Perversion |
6.6 |
6.9 |
|
Diarrhea |
6.2 |
10.3 |
|
Malaise |
6.2 |
5.3 |
|
Lower Respiratory Tract Infection |
5.8 |
8.0 |
|
Dizziness |
5.8 |
7.6 |
|
Hyperventilation |
5.4 |
9.9 |
|
Rash |
5.4 |
6.1 |
Adverse drug reactions (<5%) occurring more frequently with tobramycin inhalation solution in the placebo-controlled studies and assessed as drug-related in ≥1% of patients:
Ear and labyrinth disorders
Tinnitus (3.1%, vs 0% for placebo)
Musculoskeletal and connective tissue disorders
Myalgia (4.7%, vs 2.7% for placebo)
Infections and infestations
Laryngitis (4.3%, vs 3.1% for placebo)
Adverse drug reactions derived from spontaneous reports
The following adverse reactions have been identified during postapproval use of tobramycin inhalation solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Ear and labyrinth disorders
Hearing loss (see WARNINGS–Ototoxicity)
Skin and subcutaneous tissue disorders
Hypersensitivity, pruritus, urticaria, rash
Nervous system disorders
Aphonia, dysgeusia
Respiratory, thoracic, and mediastinal disorders
Bronchospasm (see WARNINGS–Bronchospasm), oropharyngeal pain, sputum increased, chest pain
Metabolism and Nutrition Disorders
Decreased appetite
OVERDOSAGE
Signs and symptoms of acute toxicity from overdosage of intravenous (IV) tobramycin might include dizziness, tinnitus, vertigo, loss of high-tone hearing acuity, respiratory failure, neuromuscular blockade, and renal impairment. Administration by inhalation results in low systemic bioavailability of tobramycin. Tobramycin is not significantly absorbed following oral administration. Tobramycin serum concentrations may be helpful in monitoring overdosage.
In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment. In the case of any overdosage, the possibility of drug interactions with alterations in drug disposition should be considered.
DOSAGE AND ADMINISTRATION
The recommended dosage for both adults and pediatric patients 6 years of age and older is 1 single-use ampule (300 mg) administered BID for 28 days. Dosage is not adjusted by weight. All patients should be administered 300 mg BID. The doses should be taken as close to 12 hours apart as possible; they should not be taken less than 6 hours apart.
Tobramycin inhalation solution is inhaled while the patient is sitting or standing upright and breathing normally through the mouthpiece of the nebulizer. Nose clips may help the patient breathe through the mouth.
Tobramycin inhalation solution is administered BID in alternating periods of 28 days. After 28 days of therapy, patients should stop tobramycin inhalation solution therapy for the next 28 days, and then resume therapy for the next 28 day on/28 day off cycle.
Tobramycin inhalation solution is supplied as a single-use ampule and is administered by inhalation, using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor. Tobramycin inhalation solution is not for subcutaneous, intravenous or intrathecal administration.
Usage
Tobramycin inhalation solution is administered by inhalation over an approximately 15-minute period, using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor. Tobramycin inhalation solution should not be diluted or mixed with dornase alfa (PULMOZYME, Genentech) or other medications in the nebulizer.
During clinical studies, patients on multiple therapies were instructed to take them first, followed by tobramycin inhalation solution.
HOW SUPPLIED
Tobramycin Inhalation Solution, USP 300 mg is available as follows:
NDC 0781-7171-56
5 mL single-dose ampule (carton of 56)
Storage
Tobramycin inhalation solution should be stored under refrigeration at 2ºC-8ºC/36ºF-46ºF. Upon removal from the refrigerator, or if refrigeration is unavailable, tobramycin inhalation solution pouches (opened or unopened) may be stored at room temperature (up to 25ºC/77ºF) for up to 28 days. Tobramycin inhalation solution should not be used beyond the expiration date stamped on the ampule when stored under refrigeration (2ºC-8ºC/36ºF-46ºF) or beyond 28 days when stored at room temperature (25ºC/77ºF).
Tobramycin inhalation solution ampules should not be exposed to intense light. The solution in the ampule is slightly yellow, but may darken with age if not stored in the refrigerator; however, the color change does not indicate any change in the quality of the product as long as it is stored within the recommended storage conditions.
CLINICAL STUDIES
Two identically designed, double-blind, randomized, placebo-controlled, parallel group, 24-week clinical studies (Study 1 and Study 2) at a total of 69 cystic fibrosis centers in the United States were conducted in cystic fibrosis patients with P. aeruginosa. Subjects who were less than 6 years of age, had a baseline creatinine of >2 mg/dL, or had Burkholderia cepacia isolated from sputum were excluded. All subjects had baseline FEV1 % predicted between 25% and 75%. In these clinical studies, 258 patients received tobramycin inhalation solution therapy on an outpatient basis (see Table 2) using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor.
|
Cycle 1 |
Cycle 2 |
Cycle 3 |
||||
|
28 days |
28 days |
28 days |
28 days |
28 days |
28 days |
|
|
Tobramycin |
Tobramycin |
No drug |
Tobramycin |
No drug |
Tobramycin |
No drug |
|
Placebo |
placebo |
No drug |
placebo |
No drug |
placebo |
No drug |
All patients received either tobramycin inhalation solution or placebo (saline with 1.25 mg quinine for flavoring) in addition to standard treatment recommended for cystic fibrosis patients, which included oral and parenteral anti-pseudomonal therapy, β2-agonists, cromolyn, inhaled steroids, and airway clearance techniques. In addition, approximately 77% of patients were concurrently treated with dornase alfa (PULMOZYME, Genentech).
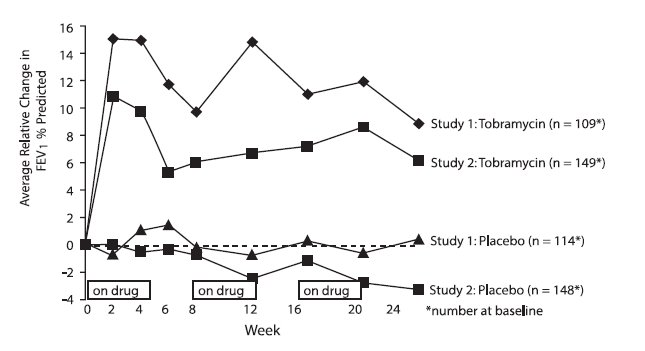
In each study, tobramycin inhalation solution-treated patients experienced significant improvement in pulmonary function. Improvement was demonstrated in the tobramycin inhalation solution group in Study 1 by an average increase in FEV1 % predicted of about 11% relative to baseline (Week 0) during 24 weeks compared to no average change in placebo patients. In Study 2, tobramycin inhalation solution-treated patients had an average increase of about 7% compared to an average decrease of about 1% in placebo patients. Figure 1 shows the average relative change in FEV1% predicted over 24 weeks for both studies.
Figure 1: Relative Change From Baseline in FEV1% Predicted
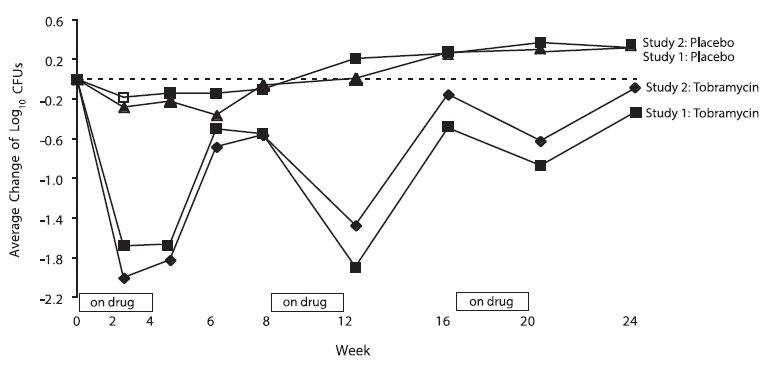
In each study, tobramycin inhalation solution therapy resulted in a significant reduction in the number of P. aeruginosa colony forming units (CFUs) in sputum during the on-drug periods. Sputum bacterial density returned to baseline during the off-drug periods. Reductions in sputum bacterial density were smaller in each successive cycle (see Figure 2).
Figure 2: Absolute Change From Baseline in Log10 CFUs
Patients treated with tobramycin inhalation solution were hospitalized for an average of 5.1 days compared to 8.1 days for placebo patients. Patients treated with tobramycin inhalation solution required an average of 9.6 days of parenteral anti-pseudomonal antibiotic treatment compared to 14.1 days for placebo patients. During the 6 months of treatment, 40% of tobramycin inhalation solution patients and 53% of placebo patients were treated with parenteral anti-pseudomonal antibiotics.
The relationship between in vitrosusceptibility test results and clinical outcome with tobramycin inhalation solution therapy is not clear. However, 4 tobramycin inhalation solution patients who began the clinical trial with P. aeruginosa isolates having MIC values ≥128 mcg/mL did not experience an improvement in FEV1 or a decrease in sputum bacterial density.
Treatment with tobramycin inhalation solution did not affect the susceptibility of the majority of P. aeruginosa isolates during the 6-month studies. However, some P. aeruginosa isolates did exhibit increased tobramycin MICs. The percentage of patients with P. aeruginosa isolates with tobramycin MICs ≥16 mcg/mL was 13% at the beginning, and 23% at the end of 6 months of the tobramycin inhalation solution regimen.
REFERENCES
1. Neu HC. Tobramycin: an overview. [Review]. J Infect Dis 1976; Suppl 134:S3-19.
2. Weber A, Smith A, Williams-Warren J et al. Nebulizer delivery of tobramycin to the lower respiratory tract. Pediatr Pulmonol 1994; 17 (5):331-9.
3. Bryan LE. Aminoglycoside resistance. Bryan LE, Ed. Antimicrobial drug resistance. Orlando, FL: Academic Press, 1984: 241-77.
U.S. Patent 5,508,269; other patents pending.
Distributed by:
Sandoz Inc.
Princeton, NJ 08540
T2015-142
October 2015
| TOBRAMYCIN
tobramycin solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sandoz Inc (005387188) |