Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cephalexin for oral suspension and other antibacterial drugs, cephalexin for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Cephalexin, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7- (D-α-Amino-α-phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4S • H2O and the molecular weight is 365.41.
Cephalexin has the following structural formula:
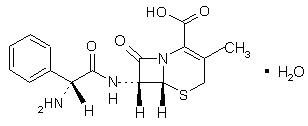
The nucleus of cephalexin is related to that of other cephalosporin antibiotics. The compound is a zwitterion; i.e., the molecule contains both a basic and an acidic group. The isoelectric point of cephalexin in water is approximately 4.5 to 5.
The crystalline form of cephalexin which is available is a monohydrate. It is a white crystalline solid having a bitter taste. Solubility in water is low at room temperature; 1 or 2 mg/mL may be dissolved readily, but higher concentrations are obtained with increasing difficulty.
The cephalosporins differ from penicillins in the structure of the bicyclic ring system.
Cephalexin has a D-phenylglycyl group as substituent at the 7-amino position and an unsubstituted methyl group at the 3-position.
Cephalexin for Oral Suspension, USP, is a pink colored powder forming pink colored suspension on constitution. After mixing, each 5 mL of cephalexin for oral suspension, will contain cephalexin USP equivalent to 125 mg (360 µmol) or 250 mg (720 µmol) of anhydrous cephalexin. The suspension also contains the following inactive ingredients: colloidal silicon dioxide, FD&C Red No. 40, sodium benzoate, strawberry flavor, sucrose, xanthan gum.
CLINICAL PHARMACOLOGY
Human Pharmacology – Cephalexin is acid stable and may be given without regard to meals. It is rapidly absorbed after oral administration. Following doses of 250 mg, 500 mg, and 1 g, average peak serum levels of approximately 9, 18, and 32 mcg/mL respectively were obtained at 1 hour. Measurable levels were present 6 hours after administration. Cephalexin is excreted in the urine by glomerular filtration and tubular secretion. Studies showed that over 90% of the drug was excreted unchanged in the urine within 8 hours. During this period, peak urine concentrations following the 250-mg, 500-mg, and 1-g doses were approximately 1000, 2200, and 5000 mcg/mL respectively.
Microbiology – In vitro tests demonstrate that the cephalosporins are bactericidal because of their inhibition of cell-wall synthesis. Cephalexin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobes, Gram-positive:
Staphylococcus aureus (including penicillinase-producing strains)
Staphylococcus epidermidis (penicillin-susceptible strains)
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobes, Gram-negative:
Escherichia coli
Haemophilus influenzae
Klebsiella pneumoniae
Moraxella (Branhamella) catarrhalis
Proteus mirabilis
Note - Methicillin-resistant staphylococci and most strains of enterococci ( Enterococcus faecalis [formerly Streptococcus faecalis]) are resistant to cephalosporins, including cephalexin. It is not active against most strains of Enterobacter spp., Morganella morganii, and Proteus vulgaris. It has no activity against Pseudomonas spp. or Acinetobacter calcoaceticus.
Susceptibility Tests –
Diffusion techniques: Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure1 that has been recommended for use with disks to test the susceptibility of microorganisms to cephalexin uses the 30-mcg cephalothin disk. Interpretation involves correlation of the diameter obtained in the disk test with the minimal inhibitory concentration (MIC) for cephalexin.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30-mcg cephalothin disk should be interpreted according to the following criteria:
| Zone Diameter (mm)
| Interpretation
|
| >18 | (S) Susceptible |
| 15-17 | (I) Intermediate |
| <14 | (R) Resistant |
A report of "Susceptible" indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in blood. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and that other therapy should be selected.
Measurement of MIC or MBC and achieved antimicrobial compound concentrations may be appropriate to guide therapy in some infections. (See CLINICAL PHARMACOLOGY section for information on drug concentrations achieved in infected body sites and other pharmacokinetic properties of this antimicrobial drug product.)
Standardized susceptibility test procedures require the use of laboratory control microorganisms.
The 30-mcg cephalothin disk should provide the following zone diameters in these laboratory test quality control strains:
| Microorganism
| Zone Diameter (mm)
|
| E. coli ATCC 25922 | 15-21 |
| S. aureus ATCC 25923 | 29-37 |
Dilution techniques: Quantitative methods that are used to determine MICs provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure uses a standardized dilution method2 (broth, agar, microdilution) or equivalent with cephalothin powder. The MIC values obtained should be interpreted according to the following criteria:
| MIC (mcg/mL)
| Interpretation
|
| <8 | (S) Susceptible |
| 16 | (I) Intermediate |
| >32 | (R) Resistant |
Interpretation should be as stated above for results using diffusion techniques.
As with standard diffusion techniques, dilution methods require the use of laboratory control microorganisms. Standard cephalothin powder should provide the following MIC values:
| Microorganism
| MIC (mcg /mL)
|
| E. coli ATCC 25922 | 4-16 |
| S. aureus ATCC 29213 | 0.12-0.5 |
INDICATIONS AND USAGE
Cephalexin is indicated for the treatment of the following infections when caused by susceptible strains of the designated microorganisms:
Respiratory tract infections caused by S. pneumoniae and S. pyogenes. (Penicillin is the usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. Cephalexin is generally effective in the eradication of streptococci from the nasopharynx; however, substantial data establishing the efficacy of cephalexin in the subsequent prevention of rheumatic fever are not available at present.)
Otitis media due to S. pneumoniae, H. influenzae, staphylococci, streptococci, and M. catarrhalis.
Skin and skin structure infections caused by staphylococci and/or streptococci.
Bone infections caused by staphylococci and/or P. mirabilis.
Genitourinary tract infections, including acute prostatitis, caused by E. coli, P. mirabilis, and K. pneumoniae.
Note – Culture and susceptibility tests should be initiated prior to and during therapy. Renal function studies should be performed when indicated.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cephalexin for oral suspension and other antibacterial drugs, cephalexin for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
Cephalexin is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
WARNINGS
BEFORE CEPHALEXIN THERAPY IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALOSPORINS AND PENICILLIN. CEPHALOSPORIN C DERIVATIVES SHOULD BE GIVEN CAUTIOUSLY TO PENICILLIN-SENSITIVE PATIENTS.
SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE EPINEPHRINE AND OTHER EMERGENCY MEASURES.
There is some clinical and laboratory evidence of partial cross-allergenicity of the penicillins and the cephalosporins. Patients have been reported to have had severe reactions (including anaphylaxis) to both drugs.
Any patient who has demonstrated some form of allergy, particularly to drugs, should receive antibiotics cautiously. No exception should be made with regard to cephalexin.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including cephalexin, and may range from mild to life threatening. Therefore, it is important to consider this diagnosis in patients with diarrhea subsequent to the administration of antibacterial agents. Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of antibiotic-associated colitis.
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
Usage in Pregnancy – Safety of this product for use during pregnancy has not been established.
PRECAUTIONS
General – Prescribing cephalexin for oral suspension in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Patients should be followed carefully so that any side effects or unusual manifestations of drug idiosyncrasy may be detected. If an allergic reaction to cephalexin occurs, the drug should be discontinued and the patient treated with the usual agents (e.g., epinephrine or other pressor amines, antihistamines, or corticosteroids).
Prolonged use of cephalexin may result in the overgrowth of non-susceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Positive direct Coombs’ tests have been reported during treatment with the cephalosporin antibiotics. In hematologic studies or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side or in Coombs’ testing of newborns whose mothers have received cephalosporin antibiotics before parturition, it should be recognized that a positive Coombs’ test may be due to the drug.
Cephalexin should be administered with caution in the presence of markedly impaired renal function. Under such conditions, careful clinical observation and laboratory studies should be made because safe dosage may be lower than that usually recommended.
Indicated surgical procedures should be performed in conjuction with antibiotic therapy.
As a result of administration of cephalexin, a false-positive reaction for glucose in the urine may occur. This has been observed with Benedict’s and Fehling’s solutions and also with Clinitest® tablets. Broad-spectrum antibiotics should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Information for Patients
Patients should be counseled that antibacterial drugs including cephalexin for oral suspension should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cephalexin for oral suspension is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cephalexin for oral suspension or other antibacterial drugs in the future.
Drug Interactions- Metformin – In healthy subjects given single 500 mg doses of cephalexin and metformin, plasma metformin mean Cmax and AUC increased by an average of 34 % and 24 %, respectively, and metformin mean renal clearance decreased by 14 %. No information is available about the interaction of cephalexin and metformin following multiple doses of either drug.
Although not observed in this study, adverse effects could potentially arise from co-administration of cephalexin and metformin by inhibition of tubular secretion via organic cationic transporter systems. Accordingly, careful patient monitoring and dose adjustment of metformin is recommended in patients concomitantly taking cephalexin and metformin.
Probenicid - As with other β-lactams, the renal excretion of cephalexin is inhibited by probenecid.
Usage in Pregnancy – Pregnancy Category B – The daily oral administration of cephalexin to rats in doses of 250 or 500 mg/kg prior to and during pregnancy, or to rats and mice during the period of organogenesis only, had no adverse effect on fertility, fetal viability, fetal weight, or litter size. Note that the safety of cephalexin during pregnancy in humans has not been established.
Cephalexin showed no enhanced toxicity in weanling and newborn rats as compared with adult animals. Nevertheless, because the studies in humans cannot rule out the possibility of harm, cephalexin should be used during pregnancy only if clearly needed.
Nursing Mothers – The excretion of cephalexin in the milk increased up to 4 hours after a 500-mg dose; the drug reached a maximum level of 4 mcg/mL, then decreased gradually, and had disappeared 8 hours after administration. Caution should be exercised when cephalexin is administered to a nursing woman.
Geriatric Use - Of the 701 subjects in 3 published clinical studies of cephalexin, 433 (62 %) were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see PRECAUTIONS, General)
ADVERSE REACTIONS
Gastrointestinal – Symptoms of pseudomembranous colitis may appear either during or after antibiotic treatment. Nausea and vomiting have been reported rarely. The most frequent side effect has been diarrhea. It was very rarely severe enough to warrant cessation of therapy. Dyspepsia, gastritis, and abdominal pain have also occurred. As with some penicillins and some other cephalosporins, transient hepatitis and cholestatic jaundice have been reported rarely.
Hypersensitivity – Allergic reactions in the form of rash, urticaria, angioedema, and, rarely, erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis have been observed. These reactions usually subsided upon discontinuation of the drug. In some of these reactions, supportive therapy may be necessary. Anaphylaxis has also been reported.
Other reactions have included genital and anal pruritus, genital moniliasis, vaginitis and vaginal discharge, dizziness, fatigue, headache, agitation, confusion, hallucinations, arthralgia, arthritis, and joint disorder. Reversible interstitial nephritis has been reported rarely. Eosinophilia, neutropenia, thrombocytopenia, and slight elevations in AST and ALT have been reported.
OVERDOSAGE
Signs and Symptoms – Symptoms of oral overdose may include nausea, vomiting, epigastric distress, diarrhea, and hematuria. If other symptoms are present, it is probably secondary to an underlying disease state, an allergic reaction, or toxicity due to ingestion of a second medication.
Treatment – To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient. Unless 5 to 10 times the normal dose of cephalexin has been ingested, gastrointestinal decontamination should not be necessary.
Protect the patient’s airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient’s vital signs, blood gases, serum electrolytes, etc. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient’s airway when employing gastric emptying or charcoal.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of cephalexin; however, it would be extremely unlikely that one of these procedures would be indicated.
The oral median lethal dose of cephalexin in rats is >5,000 mg/kg.
DOSAGE AND ADMINISTRATION
Cephalexin is administered orally.
Adults – The adult dosage ranges from 1 to 4 g daily in divided doses. The usual adult dose is 250 mg every 6 hours. For the following infections, a dosage of 500 mg may be administered every 12 hours: streptococcal pharyngitis, skin and skin structure infections, and uncomplicated cystitis in patients over 15 years of age. Cystitis therapy should be continued for 7 to 14 days. For more severe infections or those caused by less susceptible organisms, larger doses may be needed. If daily doses of cephalexin greater than 4 g are required, parenteral cephalosporins, in appropriate doses, should be considered.
Pediatric Patients – The usual recommended daily dosage for pediatric patients is 25 to 50 mg/kg in divided doses. For streptococcal pharyngitis in patients over 1 year of age and for skin and skin structure infections, the total daily dose may be divided and administered every 12 hours.
| Cephalexin Suspension
|
|
| Weight
| 125 mg/ 5 mL
|
| 10 kg (22 lb) | ½to 1 tsp q.i.d. |
| 20 kg (44 lb) | 1 to 2 tsp q.i.d. |
| 40 kg (88 lb) | 2 to 4 tsp q.i.d. |
| Weight
| 250 mg/ 5 mL
|
| 10 kg (22 lb) | ¼ to ½ tsp q.i.d. |
| 20 kg (44 lb) | ½ to 1 tsp q.i.d. |
| 40 kg (88 lb) | 1 to 2 tsp q.i.d. |
| or
|
|
| Weight
| 125 mg/ 5 mL
|
| 10 kg (22 lb) | 1 to 2 tsp b.i.d. |
| 20 kg (44 lb) | 2 to 4 tsp b.i.d. |
| 40 kg (88 lb) | 4 to 8 tsp b.i.d. |
| Weight
| 250 mg/ 5 mL
|
| 10 kg (22 lb) | ½ to 1 tsp b.i.d. |
| 20 kg (44 lb) | 1 to 2 tsp b.i.d. |
| 40 kg (88 lb) | 2 to 4 tsp b.i.d. |
In severe infections, the dosage may be doubled.
In the therapy of otitis media, clinical studies have shown that a dosage of 75 to 100 mg/kg/day in 4 divided doses is required.
In the treatment of β-hemolytic streptococcal infections, a therapeutic dosage of cephalexin should be administered for at least 10 days.
Directions for Mixing
For Cephalexin for Oral Suspension, USP 125 mg/5 mL:
| Bottle size
| Reconstitution directions
|
| 100 mL
| Add 66 mL of water in two portions to the dry mixture in the bottle. Shake well after each addition
|
| 200 mL
| Add 132 mL of water in two portions to the dry mixture in the bottle. Shake well after each addition.
|
For Cephalexin for Oral Suspension, USP 250 mg/5 mL:
| Bottle size
| Reconstitution directions
|
| 100 mL
| Add 66 mL of water in two portions to the dry mixture in the bottle. Shake well after each addition.
|
| 200 mL
| Add 132 mL of water in two portions to the dry mixture in the bottle. Shake well after each addition.
|
HOW SUPPLIED
Cephalexin for Oral Suspension, USP, is available in:
The 125 mg per 5 mL oral suspension* is available as follows:
100-mL Bottles NDC 68180-123-01
200-mL Bottles NDC 68180-123-02
The 250 mg per 5 mL oral suspension* is available as follows:
100-mL Bottles NDC 68180-124-01
200-mL Bottles NDC 68180-124-02
————————————————————————————————————
* After mixing, store in a refrigerator. May be kept for 14 days without significant loss of potency.
Shake well before using. Keep tightly closed.
Prior to mixing, store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
REFERENCES
- National Committee for Clinical Laboratory Standards: Performance standards for antimicrobial disk susceptibility tests-5th ed. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, 1993.
- National Committee for Clinical Laboratory Standards: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically-3rd ed. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA, 1993.