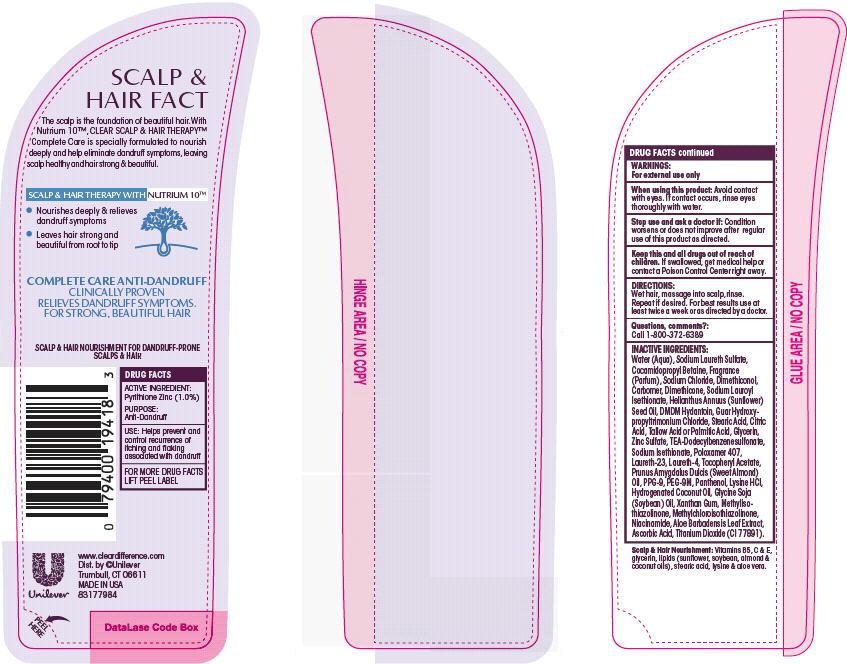
Warnings
For external use only
For external use only
When using this product: avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions: Wet hair, massage into scalp, rinse. Repeat if desired. For best results use at least twice a week or as directed by a doctor.
Inactive Ingredients
Water (Aqua)
Sodium Laureth Sulfate
Cocamidopropyl Betaine
Fragrance (Parfum)
Sodium Chloride
Dimethiconol
Carbomer
Dimethicone
Sodium Lauroyl Isethionate
Helianthus Annuus (Sunflower) Seed Oil
DMDM Hydantoin
Guar Hydroxypropyltrimonium Chloride
Stearic Acid
Citric Acid
Tallow Acid or Palmitic Acid
Zinc Sulfate
TEA-Dodecylbenzenesulfonate
Sodium Isethionate
Poloxamer 407
Laureth-23
Laureth-4
Tocopheryl Acetate
Prunus Amygdalus Dulcis (Sweet Almond) Oil
PPG-9
PEG-9M
Panthenol
Lysine HCl
Hydrogenated Coconut Oil
Glycine Soja (Soybean) Oil
Xanthan Gum
Methylisothiazolinone
Methylchloroisothiazolinone
Niacinamide
Aloe Barbadensis Leaf Extract
Ascorbic Acid
Titanium Dioxide (CI 77891)
Water (Aqua)
Sodium Laureth Sulfate
Cocamidopropyl Betaine
Fragrance (Parfum)
Sodium Chloride
Dimethiconol
Carbomer
Dimethicone
Sodium Lauroyl Isethionate
Helianthus Annuus (Sunflower) Seed Oil
DMDM Hydantoin
Guar Hydroxypropyltrimonium Chloride
Stearic Acid
Citric Acid
Tallow Acid or Palmitic Acid
Zinc Sulfate
TEA-Dodecylbenzenesulfonate
Sodium Isethionate
Poloxamer 407
Laureth-23
Laureth-4
Tocopheryl Acetate
Prunus Amygdalus Dulcis (Sweet Almond) Oil
PPG-9
PEG-9M
Panthenol
Lysine HCl
Hydrogenated Coconut Oil
Glycine Soja (Soybean) Oil
Xanthan Gum
Methylisothiazolinone
Methylchloroisothiazolinone
Niacinamide
Aloe Barbadensis Leaf Extract
Ascorbic Acid
Titanium Dioxide (CI 77891)