Warnings
Do not exceed recommended dosage.
Ask a doctor before use if the child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- diffculty in urination due to the enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Do not use this product
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- Nasal Congestion
- reduces swelling of nasal passages
- runny nose
- sneezing
- itching of the nose or throat
- itchy,watery eyes
Directions
Do Not exceed recommended dosage.
Use only with enclosed dropper. Do not use with any other device.
| Adults and Children 12 years of age and over
| 2.67 mL every 4 hours to 6 hours,not to exceed 4 doses in 24
hours |
| Children 6 years to under 12 years of age: | 1.33mL every 4 to 6 hours, not to exceed 4 doses in 24 hours |
| Children under 6 years of age:
| Consult a doctor.
|
Inactive ingredients
Citric Acid, Cotton Candy Flavor, Glycerin, Propylene Glycol, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol, Sucralose.
Questions Comments
Seek medical assistance immediately for serious side effects associated with the use of this product. Serious side effects may be reported to this number: 866-991-9870 ( mon-Fri 8 a.m to 5 p.m EST)
Product Packaging
The packaging below represents the labeling currently used.
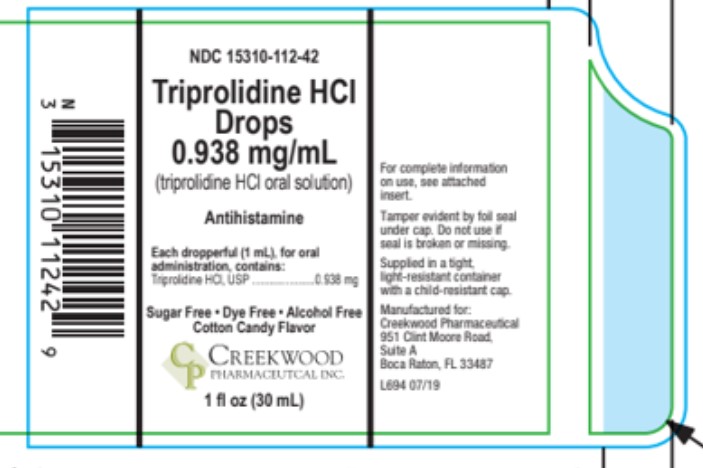
Principal display panel and side panel for 30 mL label:
NDC 15310-112-42
Triprolidine HCl Drops
Alllergic Rhintis, Respiratory Allergies & Nasal Decongestant
Common Cold
Alcohol-Free · Dye Free · Sugar Free · · Cotton Candy Flavor
Each Dropperful (1 mL) contains:
Triprolidine HCl, USP..........................0.938 mg
Net Wt. 1 FL OZ (30 mL)
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
Manufactured for:
Creekwood Pharmaceutical
951 Clint Moore Road,
Suite A
Boca Taton, FL 33487