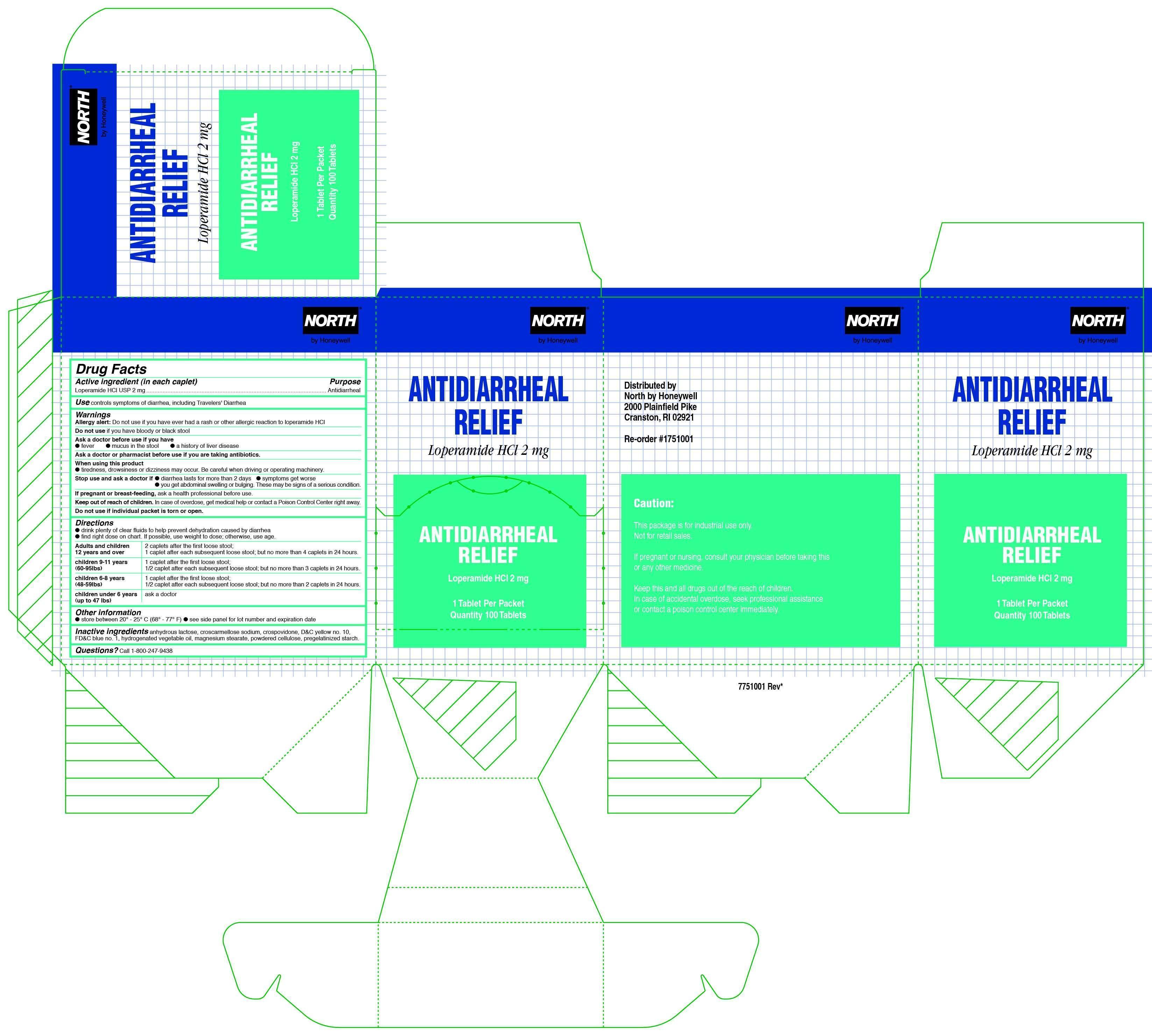
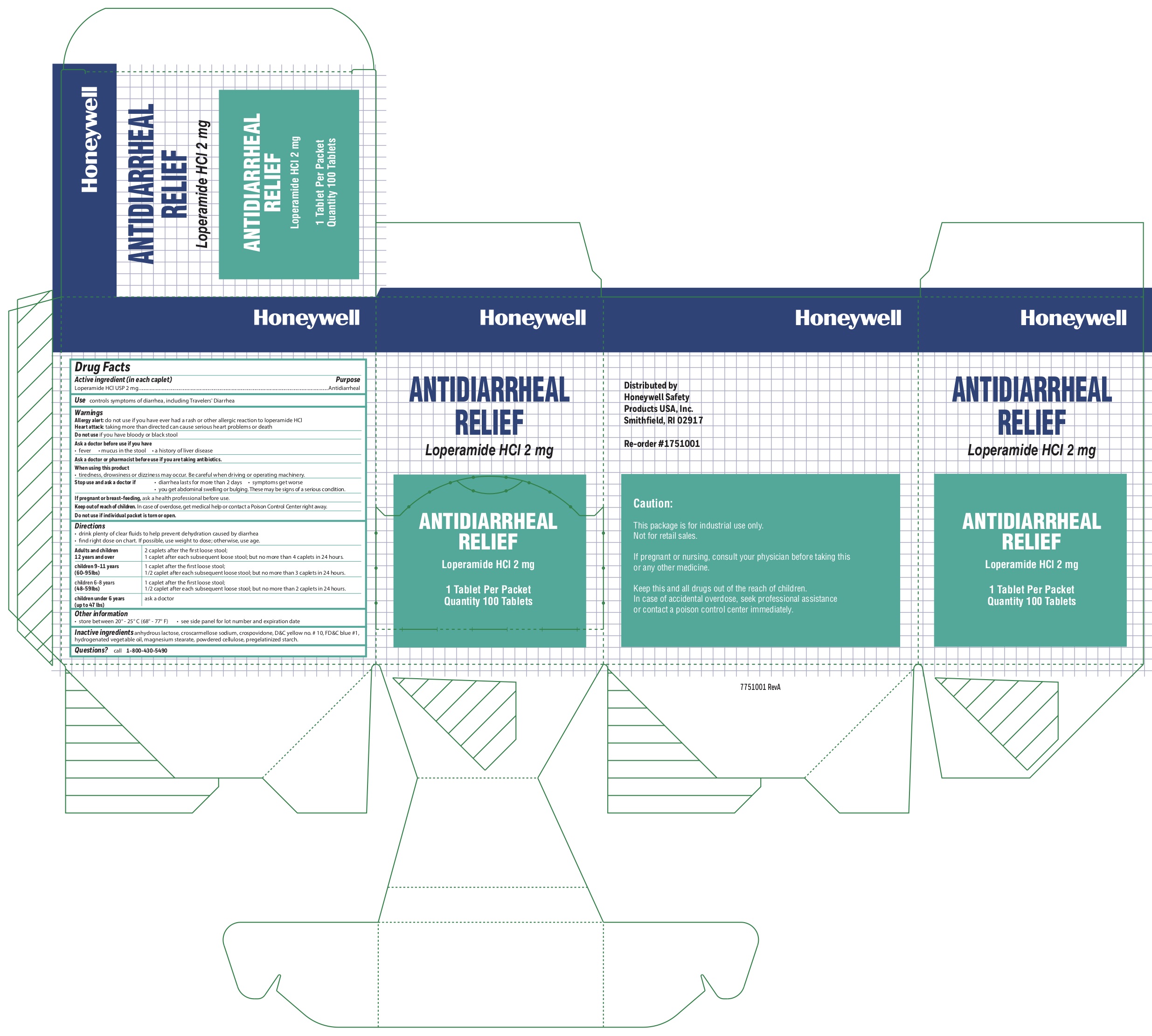
Warnings
Allergy alert: do not use if you have ever had a rash or other allergic reaction to loperamide HCl
Heart attack: taking more than directed can cause serious heart problems or death
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
Adults and children 12 years and over 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool;
but no more than 4 caplets in 24 hours.
children 9-11 years (60-95Ibs) 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours.
children 6-8 years(48-59lbs) 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours
children under 6 years (up to 47 lbs) ask a doctor