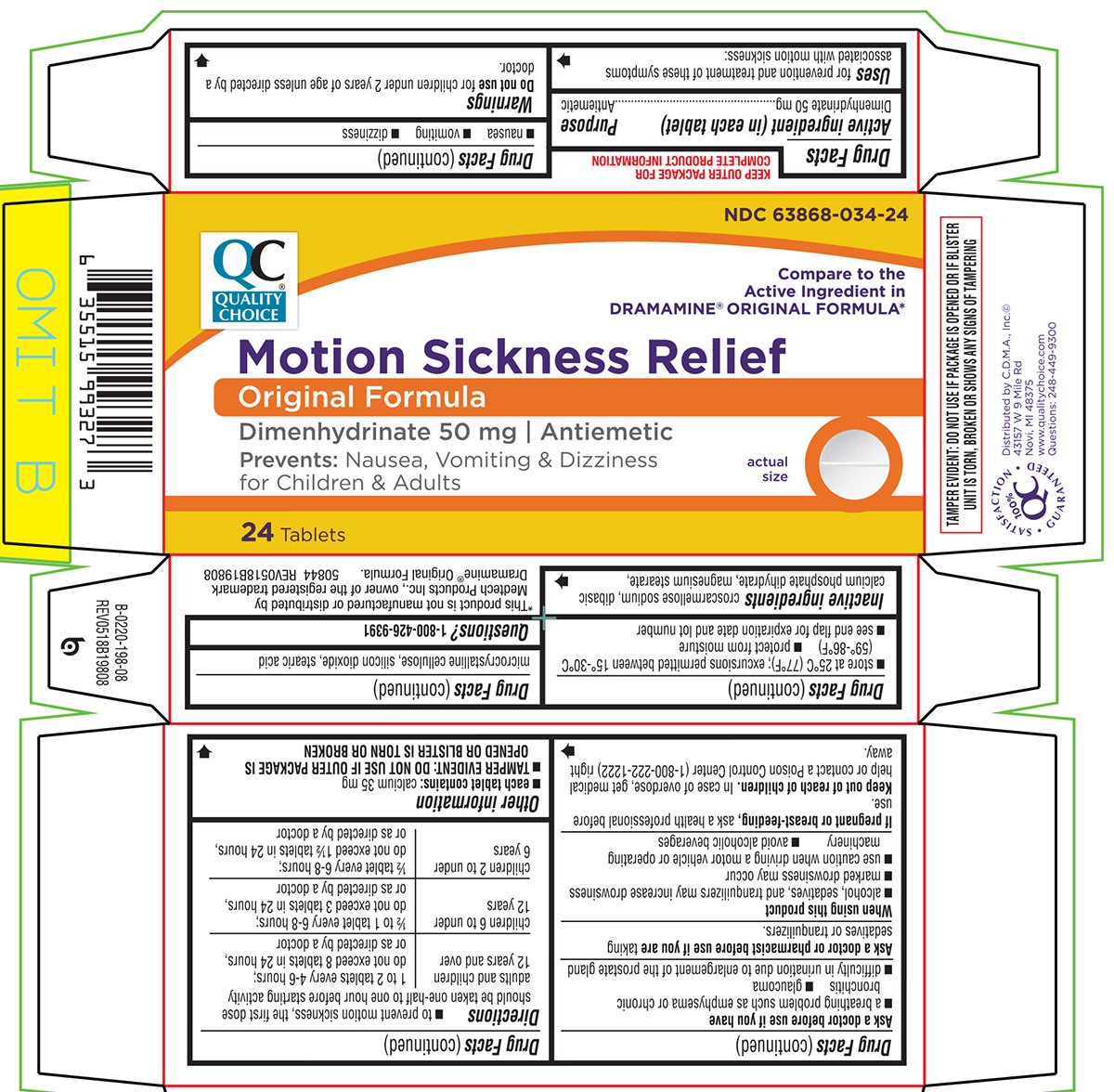
Uses
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
| adults and children 12 years and over | 1 to 2 tablets every 4-6 hours; do not exceed 8 tablets in 24 hours, or as directed by a doctor |
| children 6 to under 12 years | ½ to 1 tablet every 6-8 hours; do not exceed 3 tablets in 24 hours, or as directed by a doctor |
| children 2 to under 6 years | ½ tablet every 6-8 hours; do not exceed 1½ tablets in 24 hours, or as directed by a doctor |
Other information
-
each tablet contains: calcium 35 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid
Principal Display Panel
NDC 63868-034-12
QC®
QUALITY
CHOICE
Compare to the
Active Ingredient in
DRAMAMINE® ORIGINAL FORMULA*
Motion Sickness Relief
Original Formula
Dimenhydrinate 50 mg | Antiemetic
Prevents: Nausea, Vomiting & Dizziness
for Children & Adults
actual
size
24 Tablets
*This product is not manufactured or distributed by
Medtech Products Inc., owner of the registered trademark
Dramamine® Original Formula.
50844 REV0518B19808
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
100% QC
SATISFACTION
GUARANTEED
Distributed by C.D.M.A., Inc.©
43157 W 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions: 248-449-9300
Quality Choice 44-198