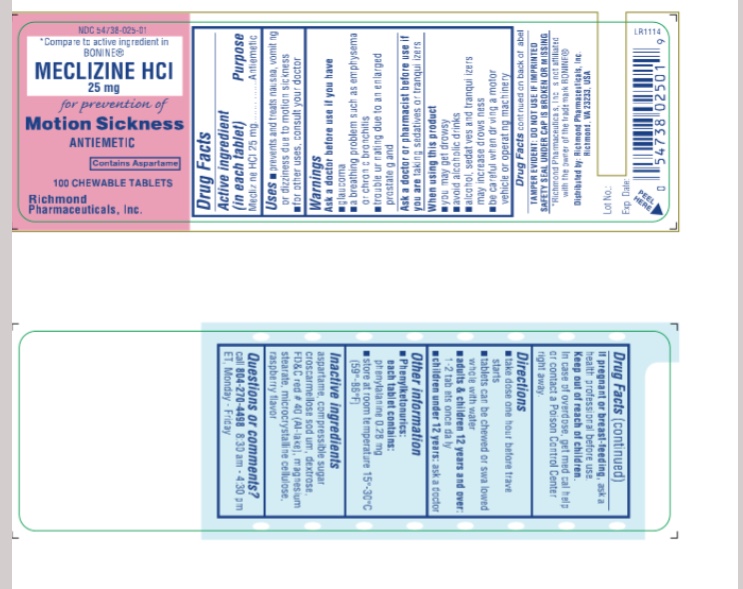
Uses
For the prevention and treatment of nausea, vomiting or dizziness associated with motion sickness. For other uses consult your doctor
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an elarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
- you may get drowsy
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increse drowsiness
- be careful when driving a moor vehicle or operating machinery
Directions
take dose one hour before travel starts
tablets can be chewed or swallowed whole with water
adults & children 12 years and over:
- take 1-2 tablets once daily
children under 12 years:
- ask a doctor
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
OTHER INFORMATION
Phenylketonurics:
each tablet contains:
phenylalanine 0.28 mg
store at room temparature 15 - 30 °C
Questions or Comments
Call 804-270-4498, 8.30 am-4.30 pm ET, Monday - Friday
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING