FULL PRESCRIBING INFORMATION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Two subcutaneous dosing options of AJOVY are available to administer the recommended dosage:
- 225 mg monthly, or
- 675 mg every 3 months (quarterly), which is administered as three consecutive subcutaneous injections of 225 mg each.
When switching dosage options, administer the first dose of the new regimen on the next scheduled date of administration. If a dose of AJOVY is missed, administer as soon as possible. Thereafter, AJOVY can be scheduled from the date of the last dose.
2.2 Important Administration Instructions
AJOVY is for subcutaneous use only.
AJOVY may be administered by healthcare professionals, patients, and/or caregivers. Prior to use, provide proper training to patients and/or caregivers on the preparation and administration of AJOVY prefilled syringe, including aseptic technique [see Instructions for Use]:
- Remove AJOVY from the refrigerator. Prior to use, allow AJOVY to sit at room temperature for 30 minutes protected from direct sunlight. Do not warm by using a heat source such as hot water or a microwave. Do not use AJOVY if it has been at room temperature for 7 days or longer [see How Supplied/Storage and Handling (16.2)].
- Follow aseptic injection technique every time AJOVY is administered.
- Inspect AJOVY for particles or discoloration prior to administration [see Dosage Forms and Strengths (3)]. Do not use if the solution is cloudy, discolored, or contains particles.
- Administer AJOVY by subcutaneous injection into areas of the abdomen, thigh, or upper arm that are not tender, bruised, red, or indurated. For multiple injections, you may use the same body site, but not the exact location of the previous injection.
- Do not co-administer AJOVY with other injectable drugs at the same injection site.
3 DOSAGE FORMS AND STRENGTHS
AJOVY is a sterile, clear to opalescent, colorless to slightly yellow solution, available as follows:
- Injection: 225 mg/1.5 mL single-dose prefilled autoinjector
- Injection: 225 mg/1.5 mL single-dose prefilled syringe
4 CONTRAINDICATIONS
AJOVY is contraindicated in patients with serious hypersensitivity to fremanezumab-vfrm or to any of the excipients. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions (5.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, pruritus, drug hypersensitivity, and urticaria, were reported with AJOVY in clinical trials. Most reactions were mild to moderate, but some led to discontinuation or required corticosteroid treatment. Most reactions were reported from within hours to one month after administration. Cases of anaphylaxis and angioedema have been reported in the postmarketing setting.
If a hypersensitivity reaction occurs, consider discontinuing AJOVY, and institute appropriate therapy [see Contraindications (4)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in clinical practice.
The safety of AJOVY was evaluated in 2512 patients with migraine who received at least 1 dose of AJOVY, representing 1279 patient-years of exposure. Of these, 1730 patients were exposed to AJOVY 225 mg monthly or AJOVY 675 mg quarterly for at least 6 months, 775 patients for at least 12 months, and 138 patients for at least 15 months. In placebo-controlled clinical trials (Studies 1 and 2), 662 patients received AJOVY 225 mg monthly for 12 weeks (with or without a loading dose of 675 mg), and 663 patients received AJOVY 675 mg quarterly for 12 weeks [see Clinical Studies (14)]. In the controlled trials, 87% of patients were female, 80% were White, and the mean age was 41 years.
The most common adverse reactions in the clinical trials for the preventive treatment of migraine (incidence at least 5% and greater than placebo) were injection site reactions. The adverse reactions that most commonly led to discontinuations were injection site reactions (1%). Table 1 summarizes adverse reactions reported in the 3-month placebo-controlled studies (Study 1 and Study 2), and the 1-month follow-up period after those studies.
| Adverse Reaction | AJOVY 225 mg Monthly (n=290) % | AJOVY 675 mg Quarterly (n=667) % | Placebo Monthly (n=668) % |
|---|---|---|---|
| Injection site reactionsa | 43 | 45 | 38 |
a Injection site reactions include multiple related adverse event terms, such as injection site pain, induration, and erythema.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to fremanezumab-vfrm in the studies described below with the incidence of antibodies in other studies to other products may be misleading. Clinical immunogenicity of AJOVY was monitored by analyzing anti-drug antibodies (ADA) and neutralizing antibodies in drug-treated patients. The data reflect the percentage of patients whose test results were positive for antibodies to AJOVY in specific assays.
In 3-month placebo-controlled studies, treatment-emergent ADA responses were observed in 6 out of 1701 (0.4%) AJOVY-treated patients. One of the 6 patients developed anti-AJOVY neutralizing antibodies at Day 84. In the ongoing long-term open-label study, ADA were detected in 1.6% of patients (30 out of 1888). Out of 30 ADA-positive patients, 17 had a neutralizing activity in their post-dose samples. Although these data do not demonstrate an impact of anti-fremanezumab-vfrm antibody development on the efficacy or safety of AJOVY in these patients, the available data are too limited to make definitive conclusions.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of AJOVY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders – Anaphylactic reactions and angioedema [see Contraindications (4) and Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AJOVY during pregnancy. Healthcare providers are encouraged to register pregnant patients, or pregnant women may enroll themselves in the registry by calling 1-833-927-2605 or visiting www.tevamigrainepregnancyregistry.com.
Risk Summary
There are no adequate data on the developmental risk associated with the use of AJOVY in pregnant women. AJOVY has a long half-life [see Clinical Pharmacology (12.3)]. This should be taken into consideration for women who are pregnant or plan to become pregnant while using AJOVY. Administration of fremanezumab-vfrm to rats and rabbits during the period of organogenesis or to rats throughout pregnancy and lactation at doses resulting in plasma levels greater than those expected clinically did not result in adverse effects on development [see Animal Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The estimated rate of major birth defects (2.2-2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data have suggested that women with migraine may be at increased risk of preeclampsia and gestational hypertension during pregnancy.
Data
Animal Data
When fremanezumab-vfrm (0, 50, 100, or 200 mg/kg) was administered to male and female rats by weekly subcutaneous injection prior to and during mating and continuing in females throughout organogenesis, no adverse embryofetal effects were observed. The highest dose tested was associated with plasma exposures (AUC) approximately 2 times that in humans at a dose of 675 mg.
Administration of fremanezumab-vfrm (0, 10, 50, or 100 mg/kg) weekly by subcutaneous injection to pregnant rabbits throughout the period of organogenesis produced no adverse effects on embryofetal development. The highest dose tested was associated with plasma AUC approximately 3 times that in humans (675 mg).
Administration of fremanezumab-vfrm (0, 50, 100, or 200 mg/kg) weekly by subcutaneous injection to female rats throughout pregnancy and lactation resulted in no adverse effects on pre- and postnatal development. The highest dose tested was associated with plasma AUC approximately 2 times that in humans (675 mg).
8.2 Lactation
Risk Summary
There are no data on the presence of fremanezumab-vfrm in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AJOVY and any potential adverse effects on the breastfed infant from AJOVY or from the underlying maternal condition.
11 DESCRIPTION
Fremanezumab-vfrm is a fully humanized IgG2Δa/kappa monoclonal antibody specific for calcitonin gene-related peptide (CGRP) ligand. Fremanezumab-vfrm is produced by recombinant DNA technology in Chinese hamster ovary (CHO) cells. The antibody consists of 1324 amino acids and has a molecular weight of approximately 148 kDa.
AJOVY (fremanezumab-vfrm) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution for subcutaneous injection, supplied in a single-dose 225 mg/1.5 mL prefilled autoinjector and a single-dose 225 mg/1.5 mL prefilled syringe.
Each prefilled autoinjector or prefilled syringe delivers 1.5 mL of solution containing 225 mg fremanezumab-vfrm, disodium ethylenediaminetetraacetic acid dihydrate (EDTA) (0.204 mg), L-histidine (0.815 mg), L-histidine hydrochloride monohydrate (3.93 mg), polysorbate-80 (0.3 mg), sucrose (99 mg), and Water for Injection, and has a pH of 5.5.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fremanezumab-vfrm is a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor.
12.2 Pharmacodynamics
The relationship between the pharmacodynamic activity and the mechanism(s) by which fremanezumab-vfrm exerts its clinical effects is unknown.
12.3 Pharmacokinetics
Absorption
After single subcutaneous (SC) administrations of 225 mg, 675 mg, and 900 mg fremanezumab-vfrm, median time to maximum concentrations (tmax) was 5 to 7 days. Dose-proportionality, based on population PK, was observed between 225 mg to 900 mg. Steady state was achieved by approximately 168 days (about 6 months) following 225 mg SC monthly and 675 mg SC quarterly dosing regimens. Median accumulation ratio, based on once-monthly and once-quarterly dosing regimens, is approximately 2.3 and 1.2, respectively.
Distribution
Fremanezumab-vfrm has an apparent volume of distribution of approximately 6 liters, suggesting minimal distribution to the extravascular tissues.
Metabolism
Similar to other monoclonal antibodies, fremanezumab-vfrm is degraded by enzymatic proteolysis into small peptides and amino acids.
Elimination
Fremanezumab-vfrm apparent clearance was approximately 0.141 L/day. Fremanezumab-vfrm was estimated to have a half-life of approximately 31 days.
Specific Populations
A population PK analysis assessing effects of age, race, sex, and weight was conducted on data from 2287 subjects. No dose adjustments are recommended for AJOVY.
Patients with Hepatic or Renal Impairment
Hepatic/renal impairment is not expected to affect the pharmacokinetics of fremanezumab. A population PK analysis of integrated data from the AJOVY clinical studies did not reveal a difference in the pharmacokinetics of fremanezumab in patients with mild hepatic impairment, relative to those with normal hepatic function. There were only 4 patients with moderate hepatic impairment, and no patient with severe hepatic impairment in fremanezumab clinical studies. No dedicated hepatic/renal impairment studies were conducted to assess the effect of hepatic or renal impairment on the pharmacokinetics of fremanezumab.
Drug Interactions
Fremanezumab is not metabolized by cytochrome P450 enzymes; therefore, interactions with concomitant medications that are substrates, inducers, or inhibitors of cytochrome P450 enzymes are unlikely. Additionally, the effects of medications for the acute treatment (specifically analgesics, ergots, and triptans) and preventive treatment of migraine were evaluated in a population PK model, and found not to influence fremanezumab exposure.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies of fremanezumab-vfrm were not conducted.
Mutagenesis
Genetic toxicology studies of fremanezumab-vfrm were not conducted.
Impairment of Fertility
When fremanezumab-vfrm (0, 50, 100, or 200 mg/kg) was administered to male and female rats by weekly subcutaneous injection prior to and during mating and continuing in females throughout organogenesis, no adverse effects on male or female fertility were observed. The highest dose tested was associated with plasma exposures (AUC) approximately 2 times that in humans at a dose of 675 mg.
14 CLINICAL STUDIES
The efficacy of AJOVY was evaluated as a preventive treatment of episodic or chronic migraine in two multicenter, randomized, 3-month, double-blind, placebo-controlled studies (Study 1 and Study 2, respectively).
Episodic Migraine
Study 1 (NCT 02629861) included adults with a history of episodic migraine (patients with <15 headache days per month). All patients were randomized (1:1:1) to receive subcutaneous injections of either AJOVY 675 mg every three months (quarterly), AJOVY 225 mg monthly, or placebo monthly, over a 3-month treatment period. Patients were allowed to use acute headache treatments during the study. A subset of patients (21%) was allowed to use one additional concomitant preventive medication.
The study excluded patients with a history of significant cardiovascular disease, vascular ischemia, or thrombotic events, such as cerebrovascular accident, transient ischemic attacks, deep vein thrombosis, or pulmonary embolism.
The primary efficacy endpoint was the mean change from baseline in the monthly average number of migraine days during the 3-month treatment period. Secondary endpoints included the proportion of patients reaching at least a 50% reduction in monthly average number of migraine days during the 3-month treatment period, the mean change from baseline in the monthly average number of days of use of any acute headache medication during the 3-month treatment period, and the mean change from baseline in the number of migraine days during the first month of the treatment period.
In Study 1, a total of 875 patients (742 females, 133 males), ranging in age from 18 to 70 years, were randomized. A total of 791 patients completed the 3-month double-blind phase. The mean migraine frequency at baseline was approximately 9 migraine days per month, and was similar across treatment groups.
Both monthly and quarterly dosing regimens of AJOVY demonstrated statistically significant improvements for efficacy endpoints compared to placebo over the 3-month period, as summarized in Table 2.
| Study 1 Efficacy Endpoint | AJOVY 225 mg Monthly (N=287) | AJOVY 675 mg Quarterly (N=288) | Placebo (N=290) |
|---|---|---|---|
| Monthly migraine days (MMD) | |||
| Baseline migraine days | 8.9 | 9.2 | 9.1 |
| Change from baseline | -3.7 | -3.4 | -2.2 |
| Difference from placebo | -1.5 | -1.2 | |
| p-value | <0.001 | <0.001 | |
| ≥50% MMD responders | |||
| % responders | 47.7% | 44.4% | 27.9% |
| Difference from placebo | 19.8% | 16.5% | |
| p-value | <0.001 | <0.001 | |
| Monthly acute headache medication days | |||
| Change from baseline | -3.0 | -2.9 | -1.6 |
| Difference from placebo | -1.4 | -1.3 | |
| p-value | <0.001 | <0.001 | |
Figure 1 displays the mean change from baseline in the average monthly number of migraine days in Study 1.
Figure 1: Change from Baseline in Monthly Migraine Days in Study 1a
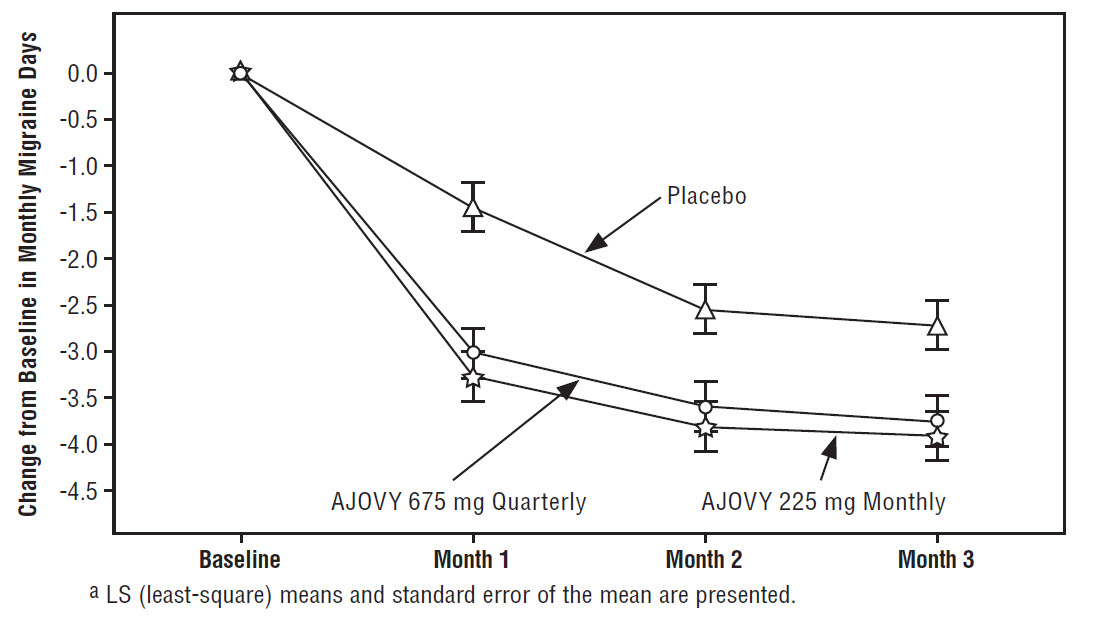
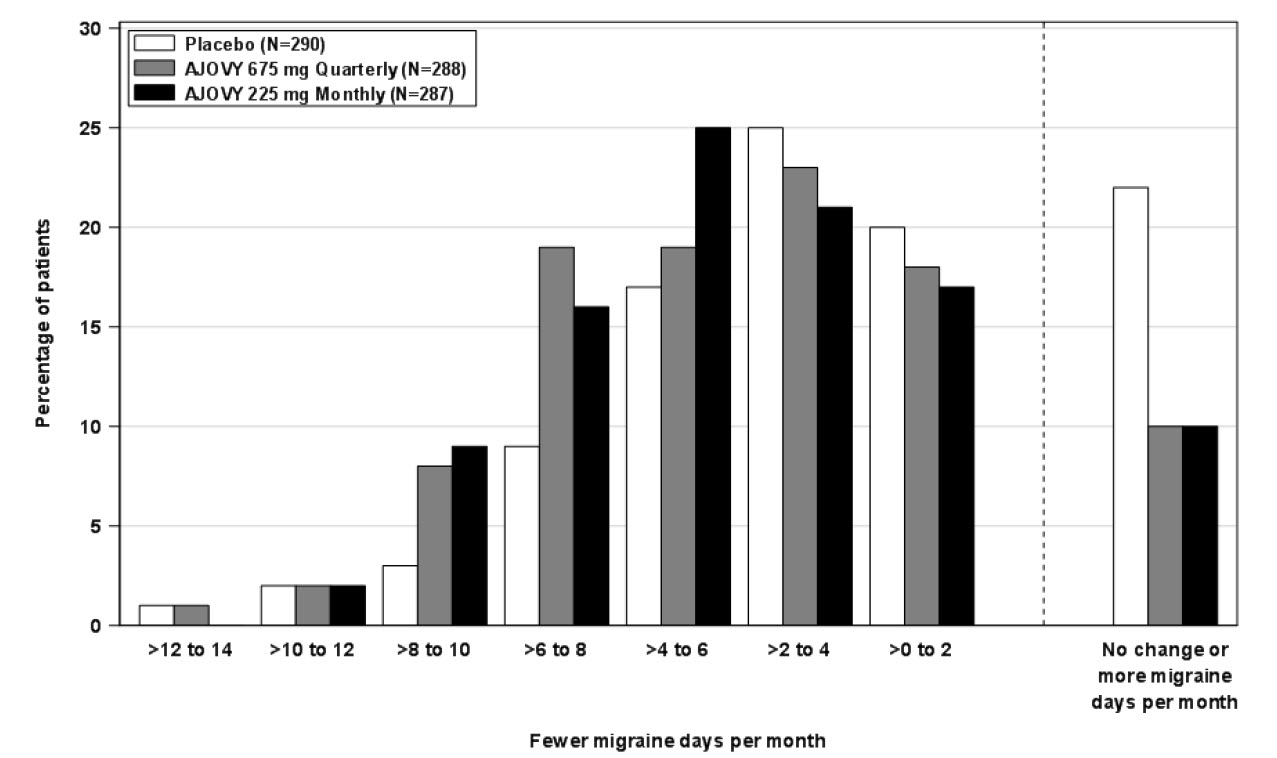
Figure 2 shows the distribution of change from baseline in mean monthly migraine days in bins of 2 days by treatment group in Study 1. A treatment benefit over placebo for both doses of AJOVY is seen across a range of changes from baseline in monthly migraine days.
Figure 2: Distribution of Change from Baseline in Mean Monthly Migraine Days by Treatment Group in Study 1
Chronic Migraine
Study 2 (NCT 02621931) included adults with a history of chronic migraine (patients with ≥15 headache days per month). All patients were randomized (1:1:1) to receive subcutaneous injections of either AJOVY 675 mg starting dose followed by 225 mg monthly, 675 mg every 3 months (quarterly), or placebo monthly, over a 3-month treatment period. Patients were allowed to use acute headache treatments during the study. A subset of patients (21%) was allowed to use one additional concomitant, preventive medication.
The study excluded patients with a history of significant cardiovascular disease, vascular ischemia, or thrombotic events, such as cerebrovascular accident, transient ischemic attacks, deep vein thrombosis, or pulmonary embolism.
The primary efficacy endpoint was the mean change from baseline in the monthly average number of headache days of at least moderate severity during the 3-month treatment period. The secondary endpoints were the mean change from baseline in the monthly average number of migraine days during the 3-month treatment period, the proportion of patients reaching at least 50% reduction in the monthly average number of headache days of at least moderate severity during the 3-month treatment period, the mean change from baseline in the monthly average number of days of use of any acute headache medication during the 3-month treatment period, and the mean change from baseline in the number of headache days of at least moderate severity during the first month of treatment.
In Study 2, a total of 1130 patients (991 females, 139 males), ranging in age from 18 to 70 years, were randomized. A total of 1034 patients completed the 3-month double-blind phase.
Both monthly and quarterly dosing regimens of AJOVY treatment demonstrated statistically significant improvement for key efficacy outcomes compared to placebo, as summarized in Table 3.
|
Study 2 |
AJOVY 225 mga
|
AJOVY 675 mg |
Placebo |
| Baseline headache days of any severityb | 20.3 | 20.4 | 20.3 |
| Baseline headache days of at least moderate severityc | 12.8 | 13.2 | 13.3 |
| Change from baseline in the monthly average number of headache days of at least moderate severity | -4.6 | -4.3 | -2.5 |
| Difference from placebo | -2.1 | -1.8 | |
| p-value | <0.001 | <0.001 | |
| Change from baseline in the monthly average number of migraine days in patients | -5.0 | -4.9 | -3.2 |
| Change from baseline in monthly average number of headache days of at least moderate severity at 4 weeks after 1st dose | -4.6 | -4.6 | -2.3 |
| Percentage of patients with ≥ 50% reduction in monthly average number of headache days of at least moderate severity | 40.8% | 37.6% | 18.1% |
| Change from baseline in monthly average number of days of acute headache medication | -4.2 | -3.7 | -1.9 |
aIn Study 2, patients received a 675 mg starting dose.
bUsed for chronic migraine diagnosis.
cUsed for primary endpoint analysis.
Figure 3 displays the mean change from baseline in the average monthly number of headache days of at least moderate severity in Study 2.
Figure 3: Change from Baseline in Monthly Headache Days of At Least Moderate Severity in Study 2a
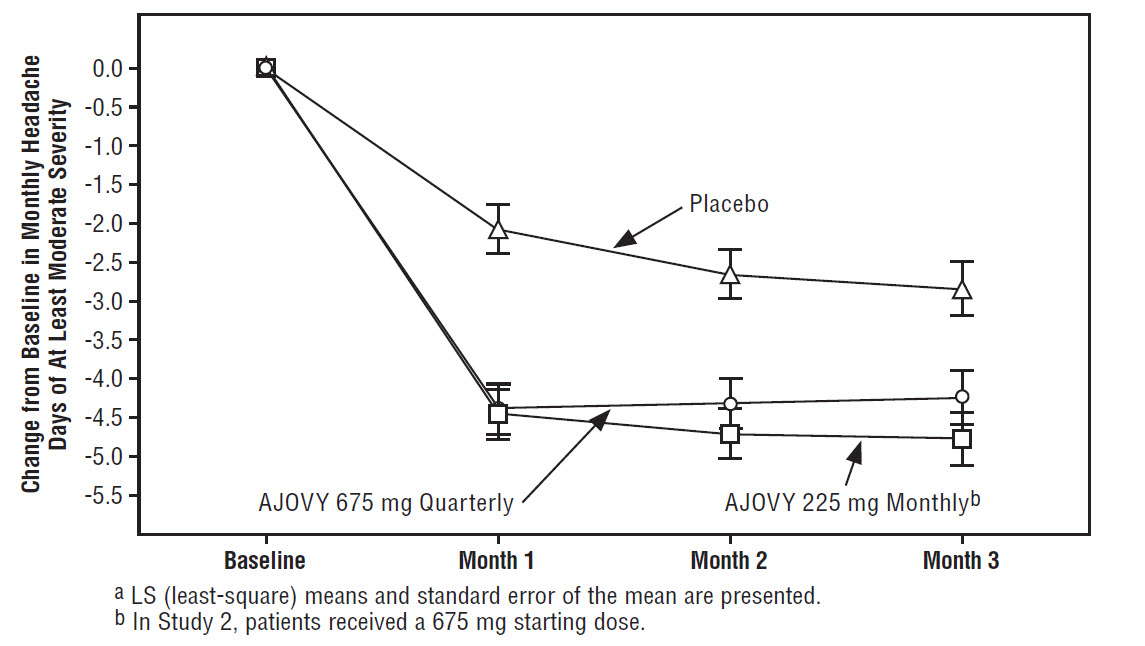
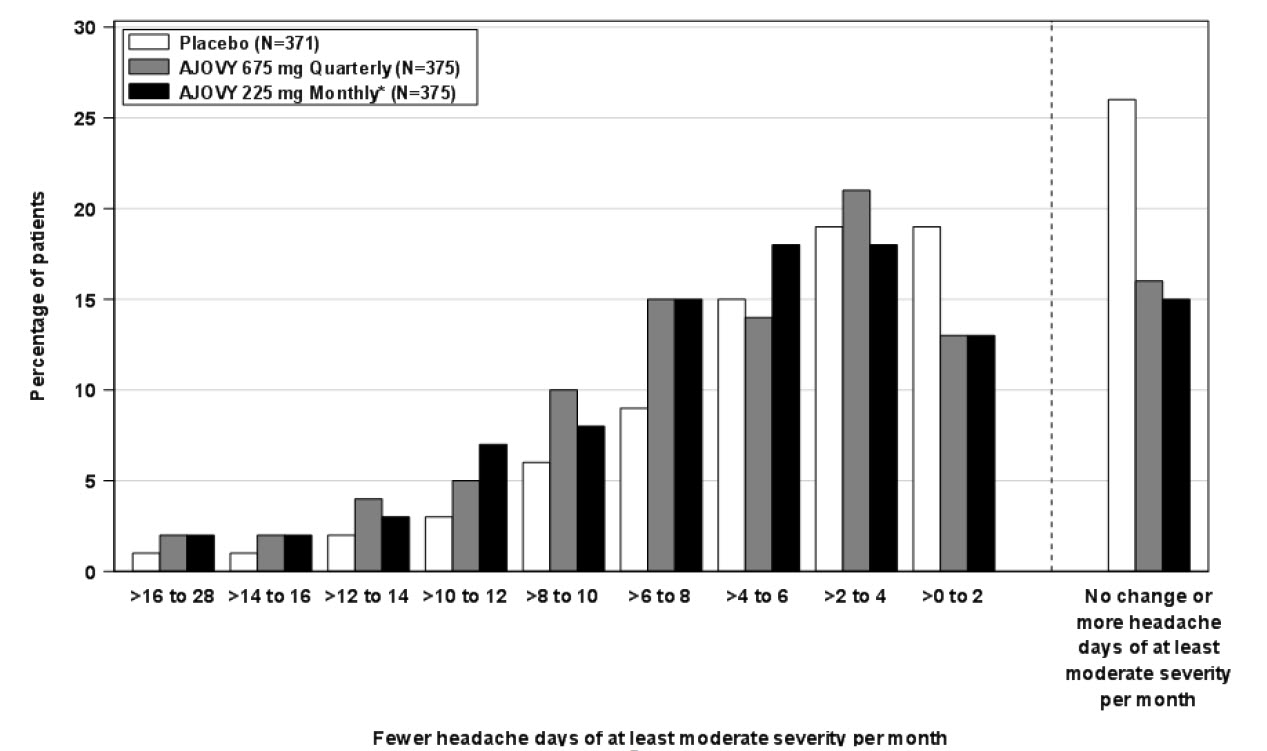
Figure 4 shows the distribution of change from baseline in monthly headache days of at least moderate severity at month 3 in bins of 3 days by treatment group. A treatment benefit over placebo for both dosing regimens of AJOVY is seen across a range of changes from baseline in headache days.
Figure 4: Distribution of Mean Change from Baseline in Monthly Headache Days of At Least Moderate Severity by Treatment Group in Study 2
*In Study 2, patients received a 675 mg starting dose.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
AJOVY (fremanezumab-vfrm) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution for subcutaneous administration.
AJOVY is not made with natural rubber latex.
AJOVY is supplied as follows:
Prefilled Autoinjector
- Pack of 1 autoinjector: 225 mg/1.5 mL single-dose prefilled autoinjector
NDC 51759-202-10 - Pack of 3 autoinjectors: 3 x 225 mg/1.5mL single-dose prefilled autoinjectors
NDC 51759-202-22
Prefilled Syringe
- Pack of 1 syringe: 225 mg/1.5 mL single-dose prefilled syringe
NDC 51759-204-10
16.2 Storage and Handling
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original outer carton to protect from light.
- If necessary, AJOVY may be kept in the original carton at room temperature up to 30°C (86°F) for a maximum of 7 days. After removal from the refrigerator, AJOVY must be used within 7 days or discarded. Once stored at room temperature, do not place back in the refrigerator.
- Do not freeze.
- Do not expose to extreme heat or direct sunlight.
- Do not shake.
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Information on Preparation and Administration
Provide guidance to patients and caregivers on proper subcutaneous administration technique, including aseptic technique, and how to use the single-dose prefilled syringe [see Dosage and Administration (2.2)]. Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use AJOVY.
Instruct patients prescribed the regimen of 675 mg every 3 months to administer the dosage as three consecutive subcutaneous injections of 225 mg each [see Dosage and Administration (2.1)].
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions and that these reactions can occur up to 1 month after administration. Advise patients to contact their healthcare provider immediately if signs or symptoms of hypersensitivity reactions occur [see Warnings and Precautions (5.1)].
Pregnancy
Advise women that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AJOVY during pregnancy [see Use in Specific Populations (8.1)].
Manufactured by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
US License No. 2016
AJOVY® (fremanezumab-vfrm), its use, or its process of manufacture, may be protected by one or more United States patents, including US 8,007,794, US 8,586,045 and US 9,896,502.
©2022 Teva Pharmaceuticals USA, Inc.
AJO-010
Patient Information
|
AJOVY® (a-JO-vee) (fremanezumab-vfrm) injection for subcutaneous use |
| What is AJOVY? AJOVY is a prescription medicine used for the preventive treatment of migraine in adults. It is not known if AJOVY is safe and effective in children. |
| Who should not use AJOVY? Do not use AJOVY if you are allergic to fremanezumab-vfrm or any of the ingredients in AJOVY. See the end of this leaflet for a complete list of the ingredients in AJOVY. |
| Before you use AJOVY, tell your healthcare provider if you:
Pregnancy Registry: There is a registry for women who become pregnant during treatment with AJOVY. The purpose of this registry is to collect information about the safety of AJOVY during pregnancy. Contact the registry as soon as you learn that you are pregnant, or ask your doctor to contact the registry for you. You or your doctor can get information and enroll you in the registry by calling 1-833-927-2605 or visiting www.tevamigrainepregnancyregistry.com.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I use AJOVY?
|
|
What are the possible side effects of AJOVY? AJOVY may cause serious side effects, including:
The most common side effects of AJOVY include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of AJOVY. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
| How should I store AJOVY?
Keep AJOVY prefilled autoinjector and AJOVY prefilled syringe out of the reach of small children. |
| General information about the safe and effective use of AJOVY. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AJOVY for a condition for which it was not prescribed. Do not give AJOVY to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about AJOVY that is written for health professionals. |
| What are the ingredients in AJOVY? Active ingredient: fremanezumab-vfrm Inactive ingredients: disodium ethylenediaminetetraacetic acid dihydrate (EDTA), L-histidine, L-histidine hydrochloride monohydrate, polysorbate-80, sucrose, and Water for Injection. AJOVY prefilled syringe and prefilled autoinjector are not made with natural rubber latex. Manufactured by: Teva Pharmaceuticals USA, Inc., North Wales, PA 19454 US License No. 2016 AJOPL-005 For more information, go to www.AJOVY.com or call 1-888-483-8279. |
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 9/2021
Instructions for Use
AJOVY® (a-JO-vee)
(fremanezumab-vfrm) injection
prefilled autoinjector, for subcutaneous use
For subcutaneous injection only.
Read and follow the Instructions for Use for your AJOVY prefilled autoinjector before you start using it and each time you get a refill.
Important:
- AJOVY prefilled autoinjector is for single-time (one-time) use only. Put AJOVY in a FDA-cleared sharps disposal or puncture-resistant container right away after use. Do not throw away (dispose of) your used sharps disposal container in your household trash.
- Before injecting, let AJOVY sit at room temperature for 30 minutes.
- Keep AJOVY prefilled autoinjector out of the reach of small children.
- After you remove the protective cap from AJOVY, to prevent infection, do not touch the needle.
- Do not inject AJOVY in your veins (intravenously).
- Do not re-use your AJOVY prefilled autoinjector as this could cause injury or infection.
- Do not share your AJOVY prefilled autoinjector with another person. You may give another person an infection or get an infection from them.
You may give AJOVY yourself. If you feel uncomfortable, you should not get your first dose of AJOVY until you or your caregiver receive training from a healthcare provider on the right way to use AJOVY.
Storage Conditions:
- Store AJOVY in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep AJOVY in the carton it comes in to protect from light.
- If needed, AJOVY may be stored at room temperature up to 86°F (30°C) in the carton it comes in for up to 7 days. Do not use AJOVY if it has been out of the refrigerator for 7 days or longer. Throw away (dispose of) AJOVY in a sharps disposal or puncture-resistant container if it has been out of the refrigerator for 7 days or longer. Once stored at room temperature, do not place back in the refrigerator.
- Do not freeze. If AJOVY freezes, throw it away in a sharps disposal container.
- Keep AJOVY out of extreme heat and direct sunlight.
Do not shake AJOVY.
AJOVY prefilled autoinjector (Before use). See Figure A.
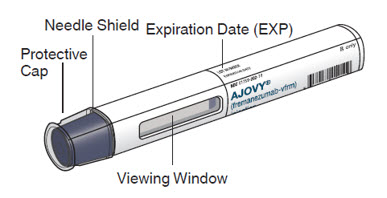
Figure A
AJOVY prefilled autoinjector (After use). See Figure B.
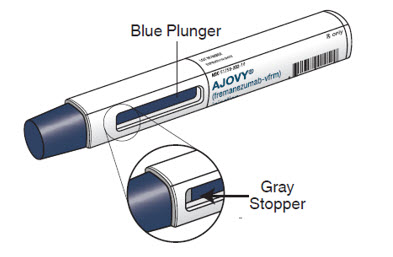
Figure B
- The blue plunger moves down the viewing window during the injection. The blue plunger fills the window when the injection is complete. Note: When the blue plunger has filled the viewing window you will still be able to see the gray stopper, as shown in Figure B.
- When injecting AJOVY, hold the prefilled autoinjector so that your hand does not cover the viewing window.
 Read this before you inject.
Read this before you inject.
Step 1. Check the dose your healthcare provider has prescribed.
AJOVY comes as a single-dose (one time) prefilled autoinjector. Your healthcare provider will prescribe the dose that is best for you.
- If your healthcare provider has prescribed 225 mg of AJOVY each month for you, give 1 injection each month, using a 225 mg prefilled AJOVY autoinjector.
- If your healthcare provider has prescribed 675 mg of AJOVY every 3 months for you, give 3 separate injections, one after another, using a different 225 mg prefilled AJOVY autoinjector for each injection. Give these injections 1 time every 3 months.
Before you inject, always check the label of your single-dose prefilled autoinjector to make sure you have the correct medicine and the correct dose of AJOVY. If you are not sure of your dose, ask your healthcare provider.
How do I inject AJOVY?
Step 2. Remove the prefilled autoinjector from the carton.
- You may need to use more than 1 prefilled autoinjector depending on your prescribed dose.
- Remove the autoinjector from the carton (see Figure C).
-
Do not shake the prefilled autoinjector at any time, as this could affect the way the medicine works.
Important: If there are any unused autoinjectors left in the carton, put the carton and unused autoinjectors back in the refrigerator.
Figure C
Step 3. Gather the supplies you will need to inject AJOVY.
- Gather the following supplies (see Figure D) and the number of AJOVY 225 mg prefilled autoinjectors you will need to give your prescribed dose:
- If your dose is 225 mg, you will need 1 AJOVY 225 mg prefilled autoinjector.
- If your dose is 675 mg, you will need 3 AJOVY 225 mg prefilled autoinjectors.
- Alcohol swabs (not supplied).
- Gauze pads or cotton balls (not supplied).
- Sharps disposal or puncture-resistant container (not supplied).

Figure D
Step 4. Let AJOVY reach room temperature.
- Place the supplies you have gathered on a clean, flat surface.
- Wait for 30 minutes to allow the medicine to reach room temperature.
- Do not leave the prefilled autoinjector in direct sunlight.
- Do not warm up the AJOVY prefilled autoinjector using a heat source such as hot water or a microwave.

Step 5. Wash your hands.
- Wash your hands with soap and water and dry well with a clean towel. Be careful not to touch your face or hair after washing your hands.
Step 6. Look closely at your AJOVY prefilled autoinjector.
Note: You may see air bubbles in the prefilled autoinjector. This is normal. Do not remove the air bubbles from the prefilled autoinjector before giving your injection.
Injecting AJOVY with these air bubbles will not harm you.
|
|
|
|
|
|
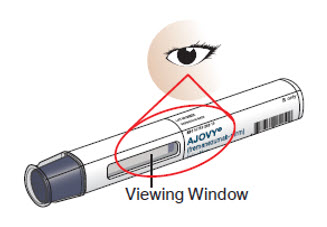
Figure E
Step 7: Choose your injection area.
-
Choose an injection area from the following areas (see Figure F):
- your stomach area (abdomen), avoid about 2 inches around the belly button.
- the front of your thighs, an area that is at least 2 inches above the knee and 2 inches below the groin.
- the back of your upper arms, in the fleshy area of the upper back portion.
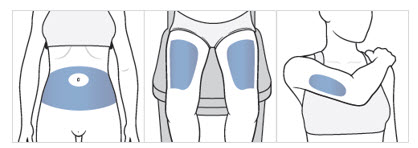
Figure F
Note: There are some injection areas on your body that are hard to reach (like the back of your arm). You may need help from someone who has been instructed on how to give your injection if you cannot reach certain injection areas.
Step 8. Clean your injection area.
- Clean the chosen injection area using a new alcohol swab. Let your skin dry.
- Do not inject AJOVY into an area that is tender, red, bruised, callused, tattooed, hard, or that has scars or stretch marks.
- Do not inject AJOVY in the same injection site that you inject other medicine.
- If you want to use the same injection area for the 3 separate injections needed for the 675 mg dose, make sure the second and third injections are not at the same spot you used for the other injections.
Step 9. Remove protective cap and do not replace.
- Pick up the prefilled autoinjector in 1 hand.
- Hold the prefilled autoinjector as shown in Figure G and pull the protective cap straight off with your other hand. Do not twist.
- Throw away the protective cap right away.
- Do not put the protective cap back on the prefilled autoinjector, to avoid injury and infection.
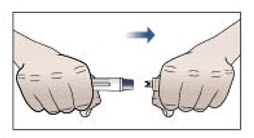
Figure G
Step 10. Give your injection.
- 10.1 Place the prefilled autoinjector at a 90 degree angle against your skin at the injection site you have cleaned (see Figure H).
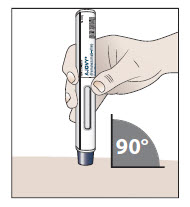
Figure H
|
10.2 Press down on the prefilled autoinjector and keep holding it down against the skin for about 30 seconds. Do not remove pressure until the 3 steps below are complete. |
||
| 1. You hear the first “click” (this means the injection has started and the blue plunger starts to move). | 2. You hear a second “click” (about 15 seconds after the first click. The plunger will be moving to the bottom of the viewing window as the medicine is being injected.) | 3. You wait another 10 seconds. (to make sure all the medicine is injected). |
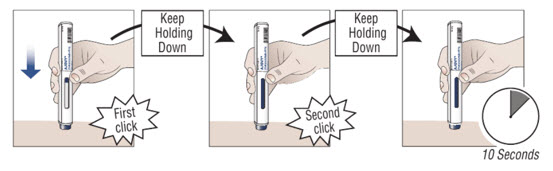 |
||
10.3 Check that the blue plunger has filled the viewing window and remove the autoinjector from the skin by lifting the prefilled autoinjector straight up (see Figure I).
Note: When the blue plunger has filled the viewing window you will be able to see the gray stopper.
As the prefilled autoinjector is lifted from the skin, the needle shield returns to the original (before use) position and locks into place, covering the needle.
Do not try to put the protective cap back on the used prefilled autoinjector as it is no longer needed.
Do not try to re-use the prefilled autoinjector.
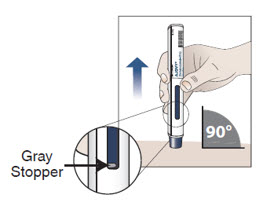
Figure I
Step 11. Apply pressure to the injection site.
- Use a clean, dry cotton ball, or gauze pad to gently press on the injection site for a few seconds.
- Do not rub the injection site.
- Do not re-use the prefilled autoinjector.
Step 12. Dispose of your prefilled autoinjector right away.
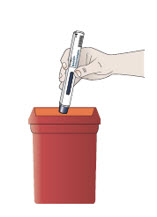
- Put your used prefilled autoinjectors in a FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) prefilled autoinjectors in your household trash. Do not recycle your used sharps disposal container.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used autoinjectors. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Injection Complete
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
US License No. 2016
©2020 Teva Pharmaceuticals USA, Inc.
AJOIFU-AI-004
Approved: 10/2020
Instructions for Use
AJOVY® (a-JO-vee)
(fremanezumab-vfrm) injection
prefilled syringe, for subcutaneous use
For subcutaneous injection only.
Read and follow the Instructions for Use for your AJOVY prefilled syringe before you start using it and each time you get a refill.
Important:
- AJOVY prefilled syringe is for single-time (one-time) use only. Put AJOVY in a FDA-cleared sharps disposal or puncture-resistant container right away after use. Do not throw away (dispose of) your used sharps disposal container in your household trash.
- Before injecting, let AJOVY sit at room temperature for 30 minutes.
- Keep AJOVY prefilled syringe out of the reach of small children.
- After you remove the needle cap from AJOVY, to prevent infection, do not touch the needle.
- Do not pull back on the plunger at any time, as this can break the prefilled syringe.
- Do not inject AJOVY in your veins (intravenously).
- Do not re-use your AJOVY prefilled syringe, as this could cause injury or infection.
- Do not share your AJOVY prefilled syringe with another person. You may give another person an infection or get an infection from them.
You may give AJOVY yourself. If you feel uncomfortable, you should not get your first dose of AJOVY until you or your caregiver receive training from a healthcare provider on the right way to use AJOVY.
Storage Conditions:
- Store AJOVY in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep AJOVY in the carton it comes in to protect from light.
- If needed, AJOVY may be stored at room temperature up to 86°F (30°C) in the carton it comes in for up to 7 days. Do not use AJOVY if it has been out of the refrigerator for 7 days or longer. Throw away (dispose of) AJOVY in a sharps disposal or puncture-resistant container if it has been out of the refrigerator for 7 days or longer. Once stored at room temperature, do not place back in the refrigerator.
- Do not freeze. If AJOVY freezes, throw it away in a sharps disposal container.
- Keep AJOVY out of extreme heat and direct sunlight.
- Do not shake AJOVY.
AJOVY prefilled syringe (Before use). See Figure A.
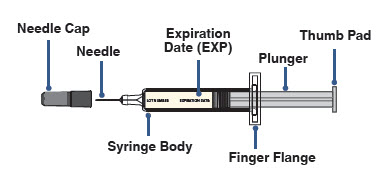
Figure A
AJOVY prefilled syringe (After use). See Figure B.

Figure B
 Read this before you inject.
Read this before you inject.
Step 1. Check the dose your healthcare provider has prescribed.
AJOVY comes as a single-dose (one time) prefilled syringe. Your healthcare provider will prescribe the dose that is best for you.
- If your healthcare provider has prescribed 225 mg of AJOVY each month for you, give 1 injection each month using a 225 mg prefilled AJOVY syringe.
- If your healthcare provider has prescribed 675 mg of AJOVY every 3 months for you, give 3 separate injections, one after another, using a different 225 mg prefilled AJOVY syringe for each injection. Give these injections 1 time every 3 months.
Before you inject, always check the label of your single-dose prefilled syringe to make sure you have the correct medicine and the correct dose of AJOVY. If you are not sure of your dose, ask your healthcare provider.
How do I inject AJOVY?
Step 2. Remove the prefilled syringe from the carton.
- You may need to use more than 1 prefilled syringe depending on your prescribed dose.
- Hold the prefilled syringe (as shown in Figure C).
- Remove the syringe from the carton.
- Do not shake the prefilled syringe at any time, as this could affect the way the medicine works.
Figure C
Step 3. Gather the supplies you will need to inject AJOVY.
- Gather the following supplies (see Figure D) and the number of AJOVY 225 mg prefilled syringes you will need to give your prescribed dose:
- If your dose is 225 mg, you will need 1 AJOVY 225 mg prefilled syringe.
- If your dose is 675 mg, you will need 3 AJOVY 225 mg prefilled syringes.
- alcohol swabs (not supplied).
- gauze pads or cotton balls (not supplied).
- sharps disposal or puncture-resistant container (not supplied).
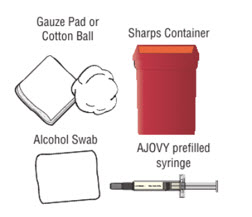
Figure D
Step 4. Let AJOVY reach room temperature.
- Place the supplies you have gathered on a clean, flat surface.
- Wait for 30 minutes to allow the medicine to reach room temperature.
- Do not leave the prefilled syringe in direct sunlight.
- Do not warm up the AJOVY prefilled syringe using a heat source such as hot water or a microwave.

Step 5. Wash your hands.
- Wash your hands with soap and water and dry well with a clean towel. Be careful not to touch your face or hair after washing your hands.
Step 6. Look closely at your AJOVY prefilled syringe.
Note: You may see air bubbles in the prefilled syringe. This is normal. Do not remove the air bubbles from the prefilled syringe before giving your injection. Injecting AJOVY with these air bubbles will not harm you.
|
|
|
|
|
|
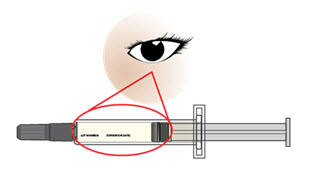
Figure E
Step 7. Choose your injection area.
- Choose an injection area from the following areas (see Figure F):
- your stomach area (abdomen), avoid about 2 inches around the belly button.
- the front of your thighs, an area that is at least 2 inches above the knee and 2 inches below the groin.
- the back of your upper arms, in the fleshy area of the upper back portion.
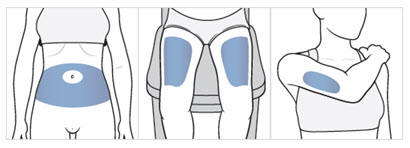
Figure F
Note: There are some injection areas on your body that are hard to reach (like the back of your arm). You may need help from someone who has been instructed on how to give your injection if you cannot reach certain injection areas.
Step 8. Clean your injection area.
-
Clean the chosen injection area using a new alcohol swab. Let your skin dry.
- Do not inject AJOVY into an area that is tender, red, bruised, callused, tattooed, hard, or that has scars or stretch marks.
- Do not inject AJOVY in the same injection site that you inject other medicine.
- If you want to use the same injection area for the 3 separate injections needed for the 675 mg dose, make sure the second and third injections are not at the same spot you used for the other injections.
Step 9. Remove needle cap and do not replace.
- Pick up the body of the prefilled syringe with 1 hand.
- Pull the needle cap straight off with your other hand (see Figure G). Do not twist.
- Throw away the needle cap right away.
- Do not put the needle cap back on the prefilled syringe, to avoid injury and infection.

Figure G
Step 10. Give your injection following the 4 steps below.
| 1. Use your free hand to gently pinch up at least 1 inch of the skin that you have cleaned. | 2. Insert the needle into the pinched skin at a 45 to 90 degree angle. | 3. When the needle is all the way into your skin, use your thumb to push the plunger. | 4. Push the plunger slowly all the way down as far as it will go to inject all of the medicine. |
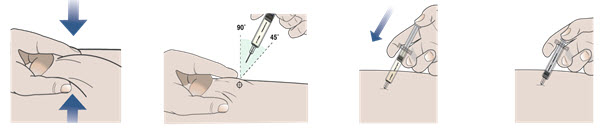 |
|||
Step 11. Remove the needle from your skin.
- After you have injected all of the medicine, pull the needle straight out (see Figure H).
- Do not recap the needle at any time to avoid injury and infection.
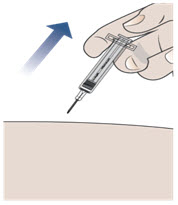
Figure H
Step 12. Apply pressure to the injection site.
- Use a clean, dry cotton ball or gauze to gently press on the injection site for a few seconds.
- Do not rub the injection site.
- Do not re-use the prefilled syringe.
Step 13. Dispose of your prefilled syringe right away.
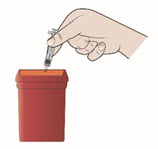
- Put your used prefilled syringes, needles, and sharps in a FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) loose needles, syringes, or prefilled syringes in your household trash. Do not recycle your used sharps disposal container.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Injection Complete
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
US License No. 2016
©2020 Teva Pharmaceuticals USA, Inc.
AJOIFU-PFS-004
Revised: 10/2020
PACKAGE/LABEL DISPLAY PANEL,Carton of 1 prefilled syringe
NDC 51759-204-10
Rx only
AJOVY® (fremanezumab-vfrm) injection 225 mg/1.5 mL
FOR SUBCUTANEOUS USE ONLY
One single-dose prefilled syringe
Store in refrigerator at 36°F to 46°F (2° to 8°C) in the original carton to protect from light. DO NOT FREEZE. DO NOT SHAKE. If needed, AJOVY® may be kept at room temperature up to 86°F (30°C) for 7 days. Once stored at room temperature, do not place back in the refrigerator. Discard after 7 days.
OPEN HERE
teva

PACKAGE/LABEL DISPLAY PANEL,Carton of 1 prefilled autoinjector
NDC 51759-202-10
Rx only
AJOVY (fremanezumab-vfrm) injection 225 mg/1.5 mL
FOR SUBCUTANEOUS USE ONLY
One single-dose prefilled autoinjector
Store in refrigerator at 36°F to 46°F (2° to 8°C) in the original carton to protect from light. DO NOT FREEZE. DO NOT SHAKE. If needed, AJOVY® may be kept at room temperature up to 86°F (30°C) for 7 days. Once stored at room temperature, do not place back in the refrigerator. Discard after 7 days.
OPEN HERE
teva

PACKAGE/LABEL DISPLAY PANEL,Carton of 3 prefilled autoinjectors
NDC 51759-202-22
Rx only
AJOVY (fremanezumab-vfrm) injection 225 mg/1.5 mL
FOR SUBCUTANEOUS USE ONLY
3 x 225 mg/1.5 mL single-dose prefilled autoinjectors
Store in refrigerator at 36°F to 46°F (2° to 8°C) in the original carton to protect from light. DO NOT FREEZE. DO NOT SHAKE. If needed, AJOVY® may be kept at room temperature up to 86°F (30°C) for 7 days. Once stored at room temperature, do not place back in the refrigerator. Discard after 7 days.
OPEN HERE
teva
