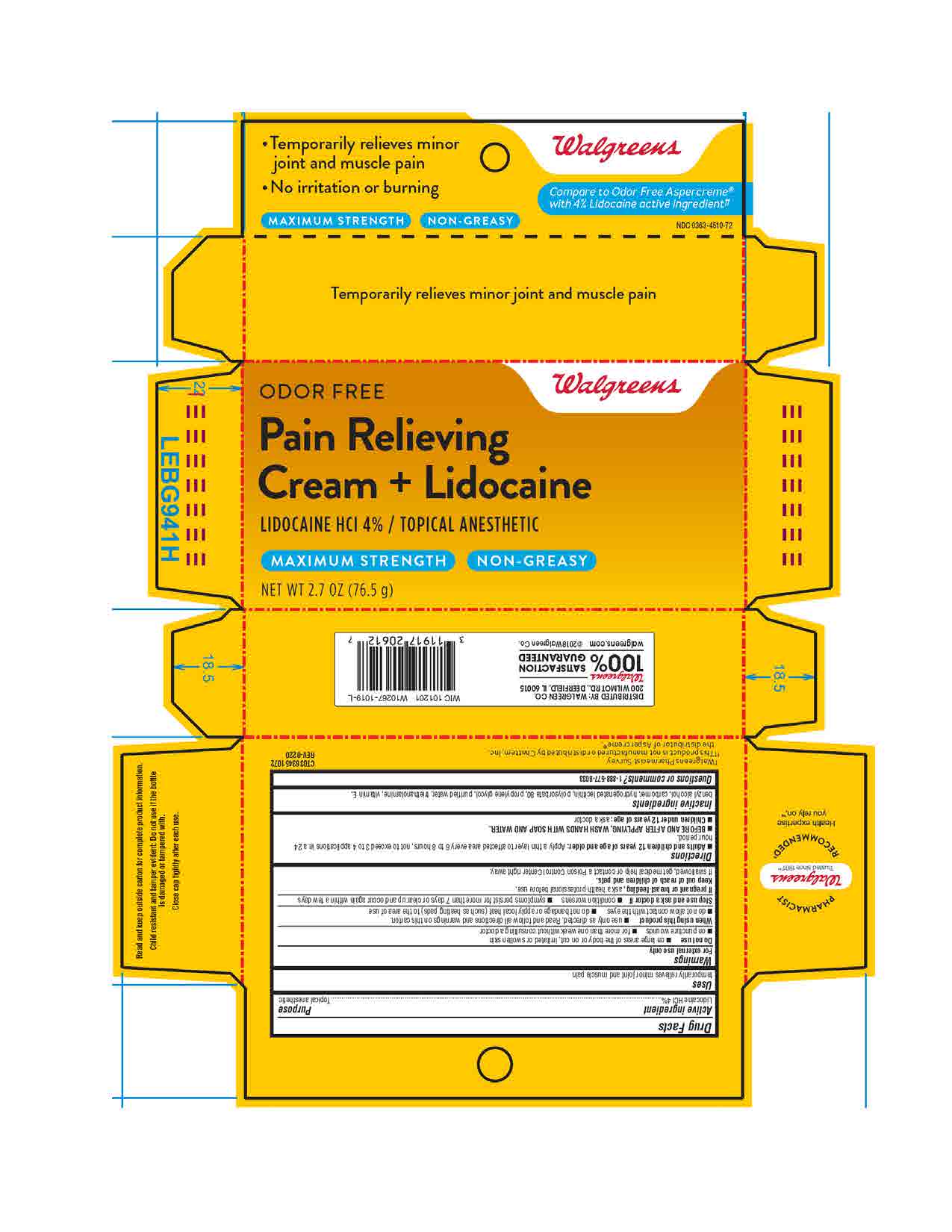
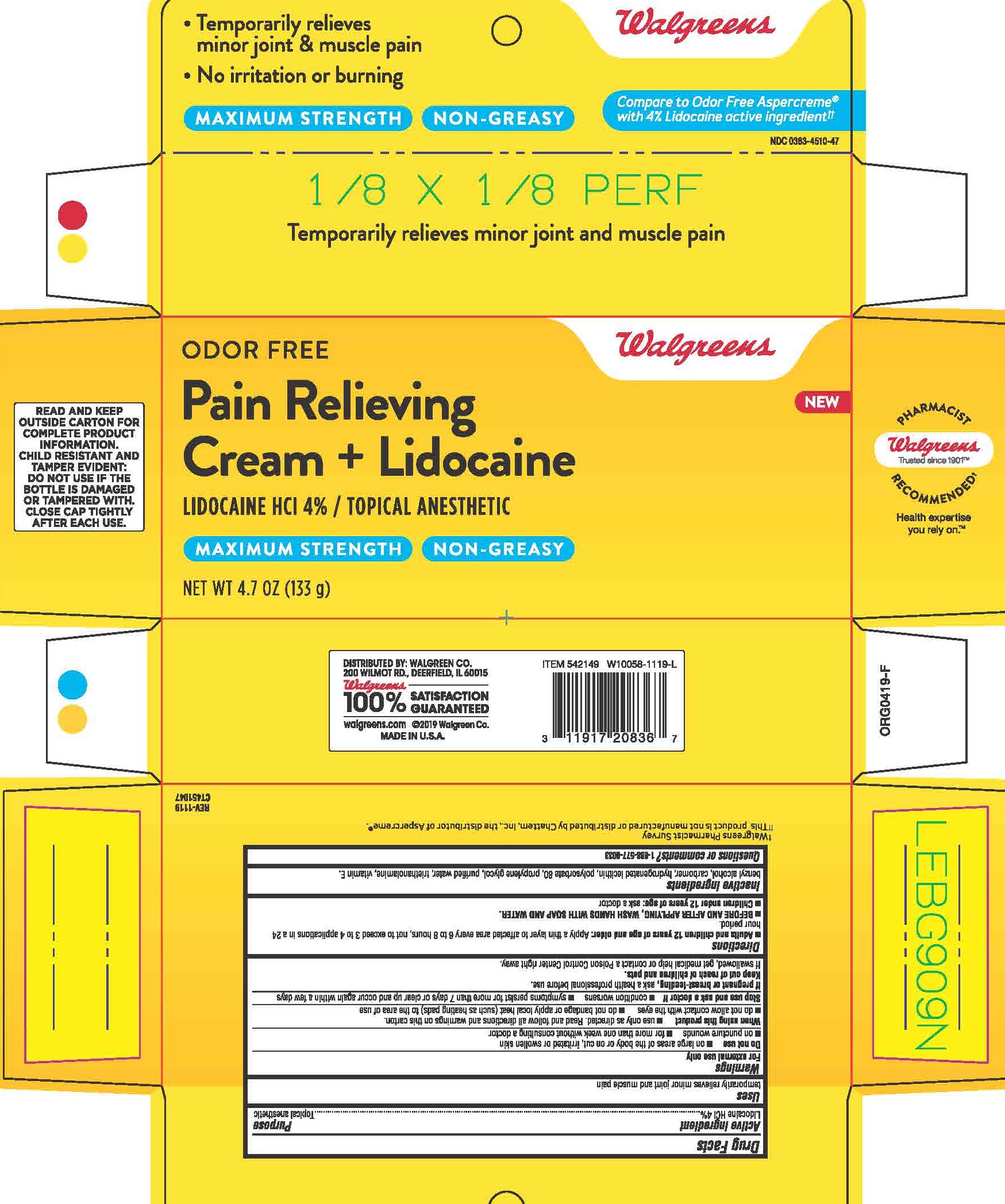
benzyl alcohol, carbomer, hydrogenated lecithin, polysorbate 80, propylene glycol, purified water, triethanolamine, vitamin E
Adults and children 12 years and older: apply a thin layer to affected area every 6 to 8 hours, not to exceed 3 to 4 applications in a 24-hour period.
BEFORE AND AFTER APPLYING, WASH HANDS WITH SOAP AND WATER.
Children under 12 years: ask a doctor
For external use only
Do not use on large areas of the body or on cut, irritated or swollen skin, on puncture wounds, for more than one week without consulting a doctor.
When using this product use only as directed. Read and follow all directions and warnings on this carton, do not allow contact with the eyes, do not bandage or apply local heat (such as heating pads) to the area of use.
Stop use and ask a doctor if condition worsens, symptoms persist for more than 7 days or clear up and occur again within a few days.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children and pets.
If swallowed, get medical help or contact a Poison Control Center right away.