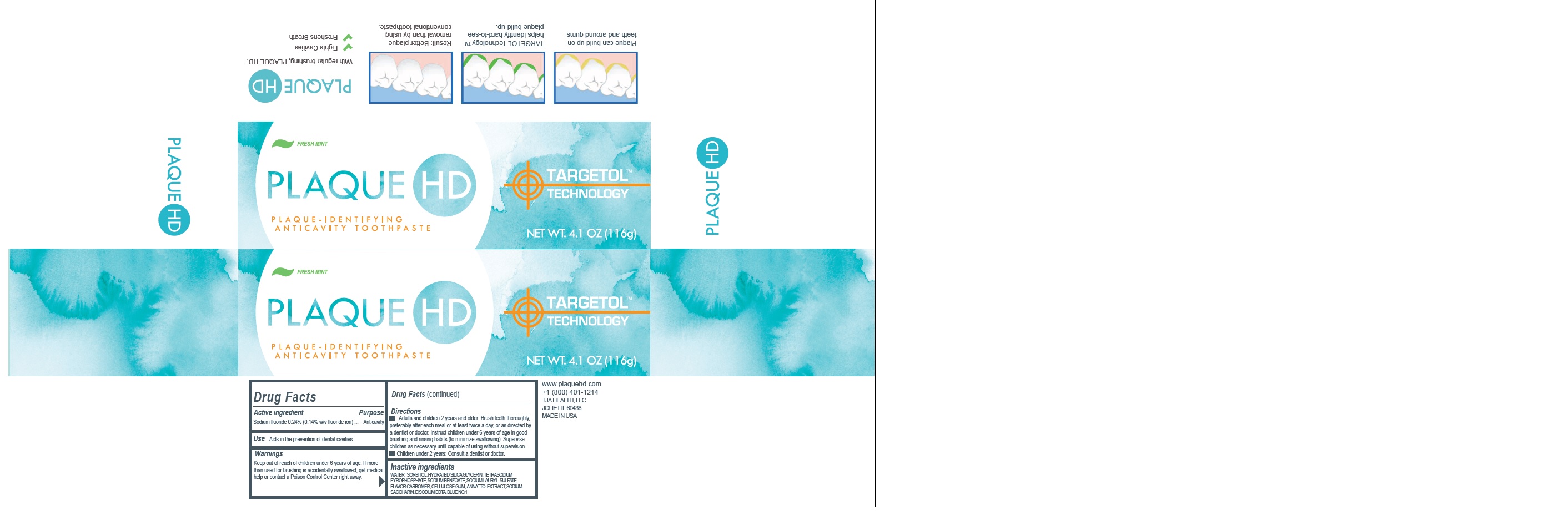
Active Ingredient
Sodium Fluoride 0.24% (0.14% w/v Fluoride Ion)
Use
Aids in the prevention of dental cavities.
Warnings
Keep out of reach of children
under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age or older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a doctor. Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision.
- Children under 2 year of age: Consult a dentist or doctor
Other information
- Store at room temperature
Inactive ingredients
Water, sorbitol, hydrated silica, glycerin, tetrasodium pyrophsphate, sodium bicarbonate, PVP, sodium benzoate, sodium lauryl sulfate, flavor, carbomer, cellulose gum, annatto extract, xylitol, sodium saccharin, disodium EDTA, blue 1.
Questions?
call toll-free-1-(800) 737-4860
Package Labeling: 57660-000-01


Representative Labeling (57660-000-03)