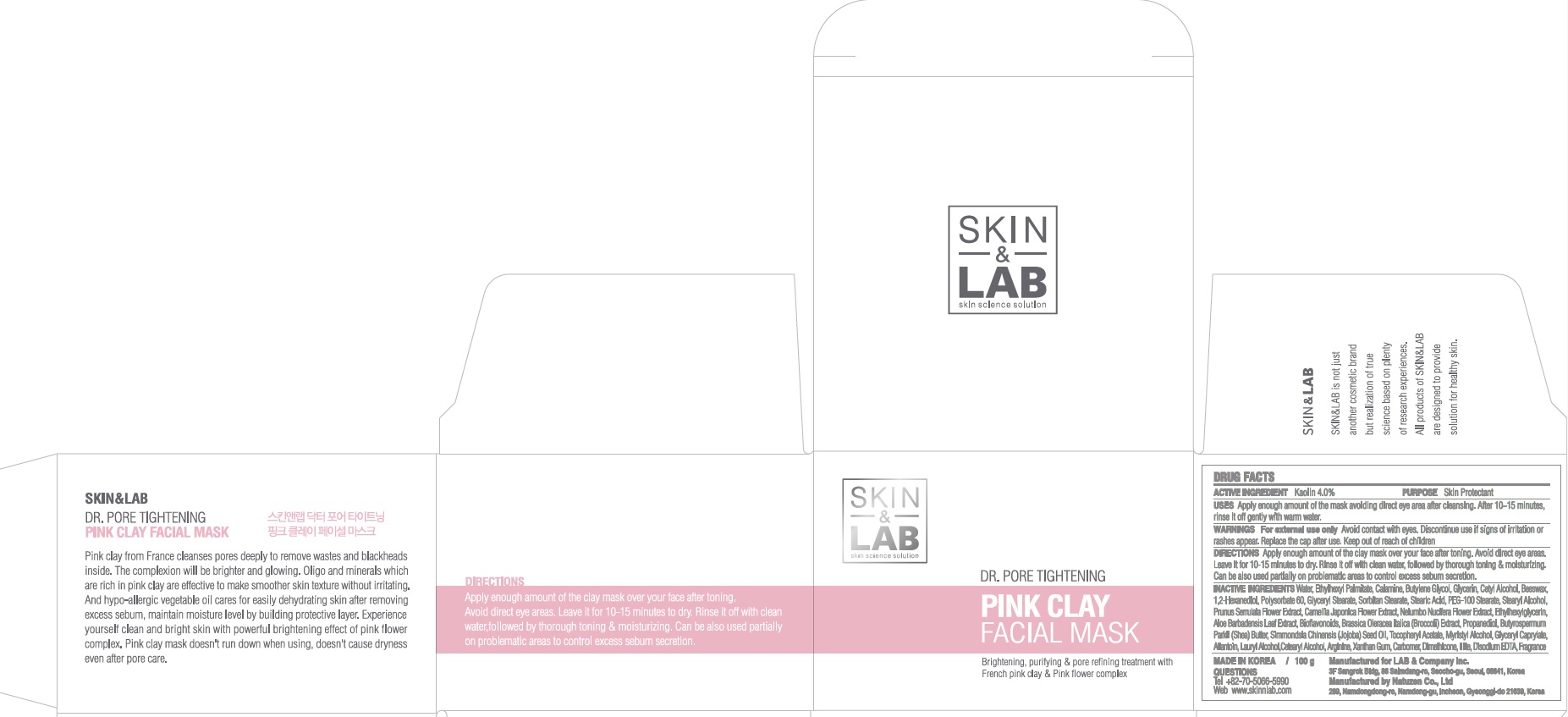
INACTIVE INGREDIENT
Inactive ingredients: Water, Ethylhexyl Palmitate, Calamine, Butylene Glycol, Glycerin, Cetyl Alcohol, Beeswax, 1,2-Hexanediol, Polysorbate 60, Glyceryl Stearate, Sorbitan Stearate, Stearic Acid, PEG-100 Stearate, Stearyl Alcohol, Prunus Serrulata Flower Extract, Camellia Japonica Flower Extract, Nelumbo Nucifera Flower Extract, Ethylhexylglycerin, Aloe Barbadensis Leaf Extract, Bioflavonoids, Brassica Oleracea Italica (Broccoli) Extract, Propanediol, Butyrospermum Parkii (Shea) Butter, Simmondsia Chinensis (Jojoba) Seed Oil, Tocopheryl Acetate, Myristyl Alcohol, Glyceryl Caprylate, Allantoin, Lauryl Alcohol, Cetearyl Alcohol, Arginine, Xanthan Gum, Carbomer, Dimethicone, Illite, Disodium EDTA, Fragrance
WARNINGS
Warnings: For external use only. Avoid contact with eyes. Discontinue use if signs of irritation or rashes appear. Replace the cap after use. Keep out of reach of children.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children: Keep out of reach of babies and children.
Uses
Uses: Apply enough amount of the mask avoiding direct eye area after cleasning. After 10~15 minutes, rinse it off gently with warm water
Directions
Directions: Apply enough amount of the clay mask over your face after toning. Avoid direct eye areas. Leave it for 10-15 minutes to dry. Rinse it off with clean water, followed by thorough toning & moisturizing. Can be also used partially on problematic areas to control excess sebum secretion.