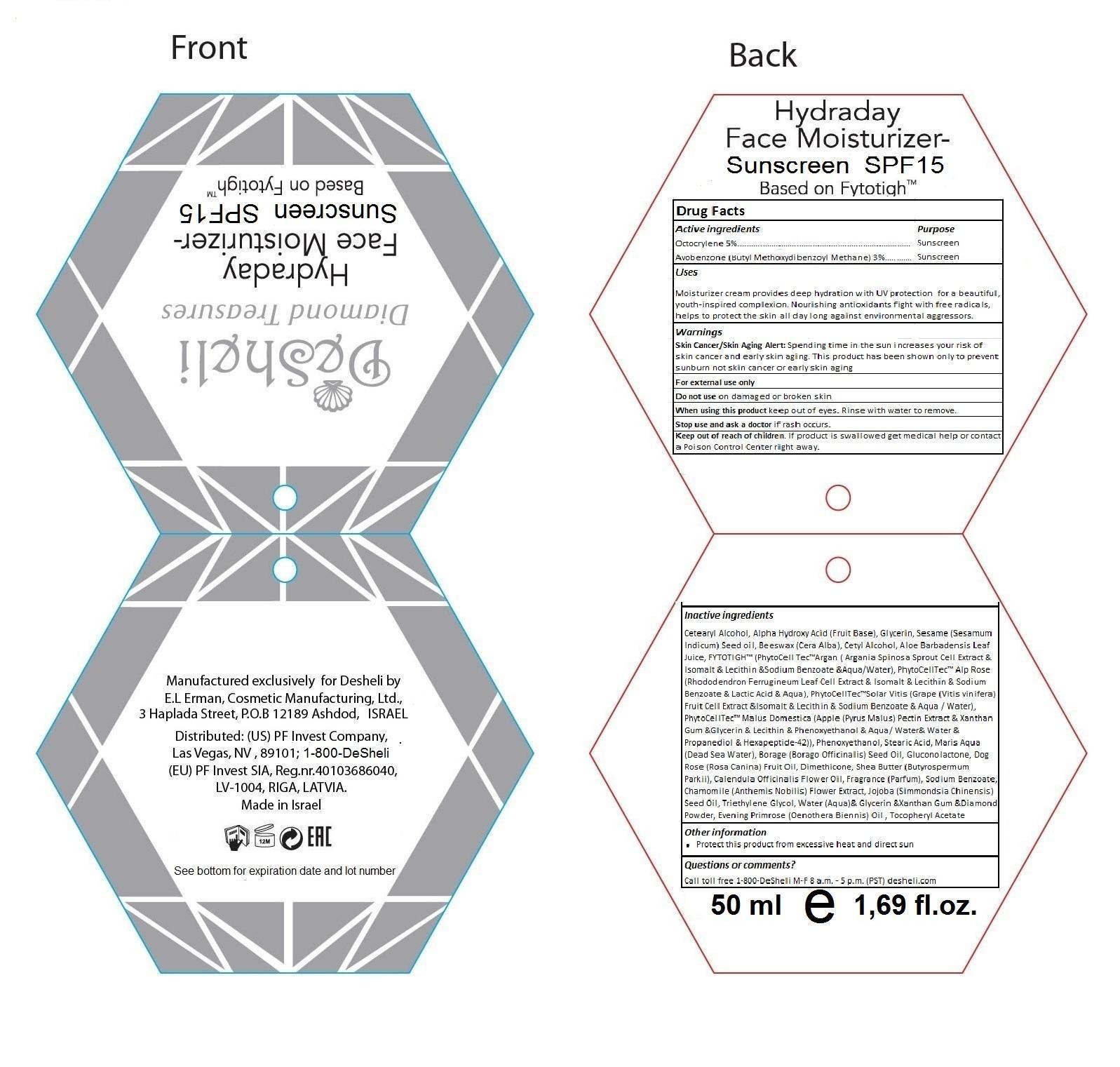
Active Ingredients Purpose
Octocrylene 5% ................................................................. Sunscreen
Avobenzone (Butyl Methoxydibenzoyl Methane) 3% ......... Sunscreen
Desheli
Diamond Treasure
Hydraday Face Moisturizer - Sunscreen SPF15
Based on Fytotigh™
Manufactured exclusively for Desheli by E.L Erman, Cosmetic Manufacturing, Ltd., 3 Haplada Street, P.O.B 12189 Ashdod, ISRAEL
Distributed: (US) PF Invest Company, Las Vegas, NV, 89101; 1-800-DeSheli (EU) PF Invest SIA, Reg.nr.40103686040, LV-1004, RIGA, LATVIA
Made in Israel
Other Information
Protect this product from excessive heat and direct sun
Questions or comments?
Call toll free 1-800-DeSheli M-F 8 a.m. - 5 p.m. (PST) desheli.com
50 ml e 1,69 fl.oz.
Keep out of reach of children. If product is swallowed get medical help or contact a Poison Control Center right away.
Uses
Moisturizer cream provides deep hydration with UV protection for a beautiful, youth-inspired complexion. Nourishing antioxidants fight with free radicals, helps to protect the skin all day long against environmental aggressors.
Warnings
Skin cancer/skin aging alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunbutn not skin cancer or early skin aging
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Uses
Moisturizer cream provides deep hydration with UV protection for a beautiful, youth-inspired complexion. Nourishing antioxidants fight with free radicals, helps to protect the skin all day long against environmental aggressors.
Inactive Ingredients
Cetearyl alcohol, alpha hydroxy acid (fruit base), glycerin, sesame (sesamum indicum) seed oil, beeswax (cera alba), cetyl alcohol, aloe barbadensis leaf juice, FYTOTIGH™ (PhytoCell Tec™Argan (argania spinosa sprout cell extract & isomalt & lecithin & sodium benzoate & aqua/water), PhytoCell Tec™Solar Vitis (Grape (vitis vinifera) fruit cell extract & isomalt & lecithin & sodium benzoate & aqua/water), PhytoCell Tec™Malus Domestica (apple (pyrus malus) pectin extract & xanthan gum & glycerin & lecithin & phenoxyethanol & aqua/water & propanediol & hexapeptide-42)), phenoxyethanol, stearic acid, maris aqua (Dead Sea water), borage (borago officinalis) seed oil, gluconolactone, dog rose (rosa canina) fruit oil, dimethicone, shea butter (butyrospermum parkii), calendula officinalis flower oil, fragrance (parfum), sodium benzoate, chamomile (anthemis nobilis) flower extract, jojoba (simmondsia chinensis) seed oil, triethylene glycol, water (aqua) & glycerin & xanthan gum & diamond powder, evening primrose (oenothera biennis) oil, tocopheryl acetate