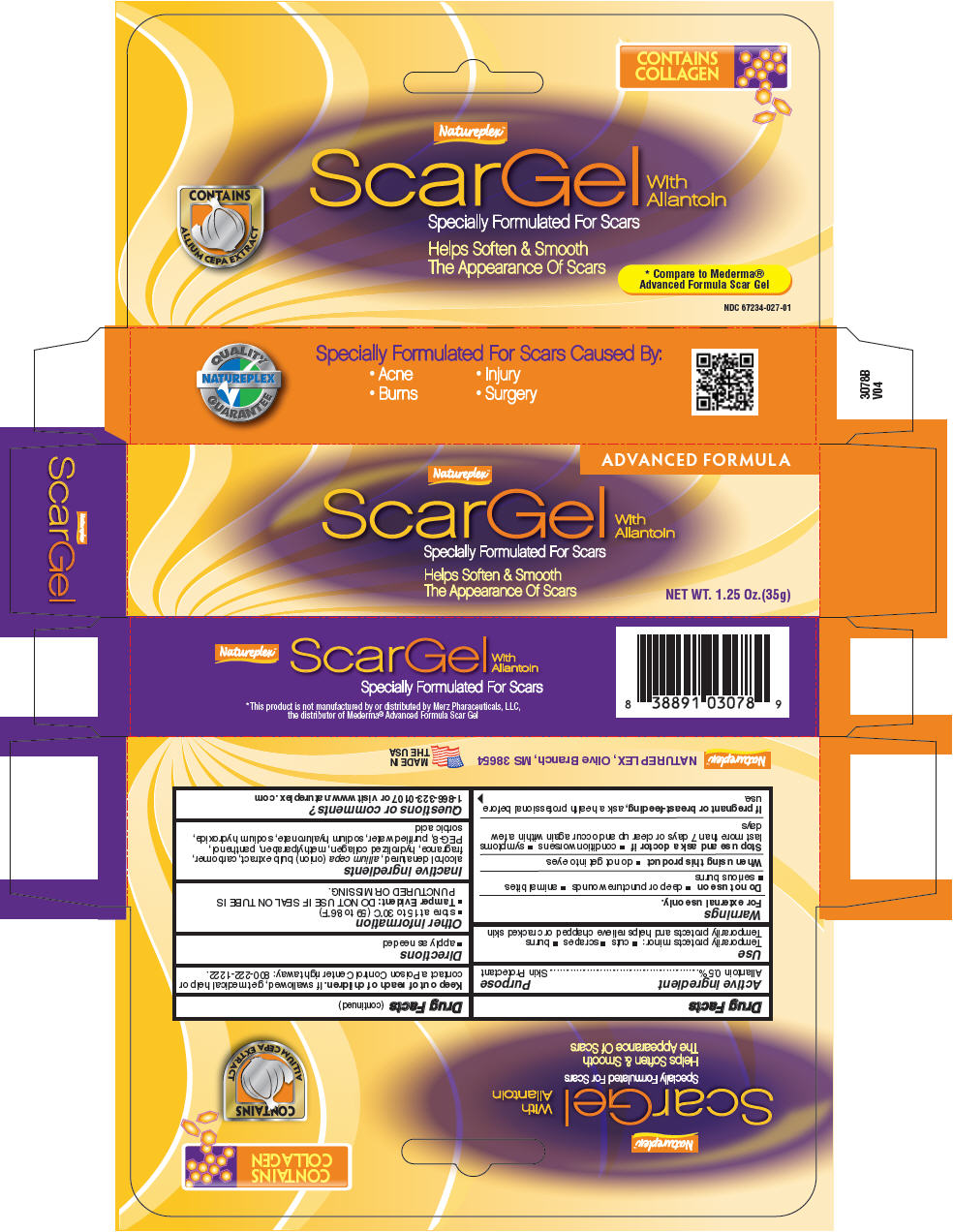
SCAR GEL ADVANCED FORMULA- allantoin cream
Natureplex LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Allantoin 0.5%
Use
Temporarily protects minor:
Temporarily protects and helps relieve chapped or cracked skin
Warnings
For external use only.
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away: 800-222-1222.
Other information
- store at 15 to 30°C (59 to 86°F)
-
Tamper Evident: DO NOT USE IF SEAL ON TUBE IS PUNCTURED OR MISSING.
Inactive ingredients
alcohol denatured, allium cepa (onion) bulb extract, carbomer, fragrance, hydrolized collagen, methylparaben, panthenol, PEG-8, purified water, sodium hyaluronate, sodium hydroxide, sorbic acid
Questions or comments?
1-866-323-0107 or visit www.natureplex .com
PRINCIPAL DISPLAY PANEL - 35 g Tube Box
ADVANCED FORMULA
Natureplex™
ScarGel
With
Allantoin
Specially Formulated For Scars
Helps Soften & Smooth
The Appearance Of Scars
NET WT. 1.25 Oz.(35g)