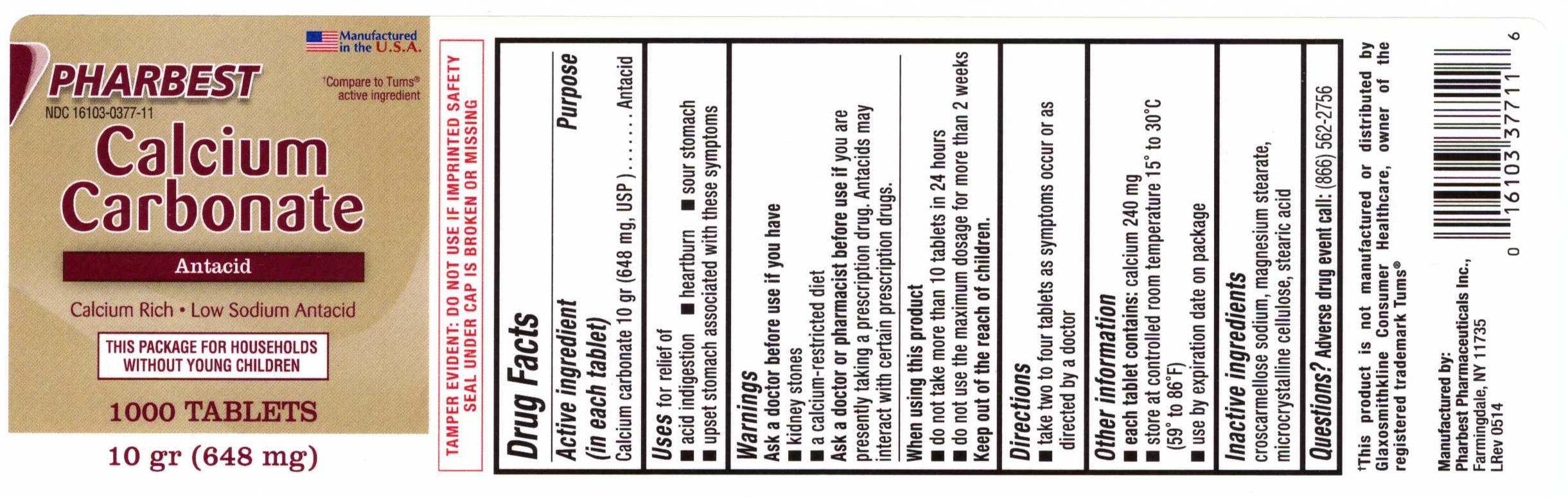
CALCIUM CARBONATE
(ANTACID)- calcium carbonate tablet
Pharbest Pharmaceuticals Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each tablet)
Calcium carbonate 10 gr (648 mg, USP)
Uses
for relief of
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
Warnings
Ask a doctor before use if you have
- kidney stone
- a calcium-restricted diet
Ask a doctor or pharmacist before use if
you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this prouct
- do not take more than 10 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks
Keep out of the reach of children.
Direction
- take two to four tablets as symptoms occur or as directed by a doctor
Other information
-
each tablet contains: calcium 240 mg
- store at controlled room temperature 150 to 300C (590 to 860F)
- use by expiration date on package
Inactive ingredients
croscarmellose sodium, magnesium stearate, microcrystalline cellulose, stearic acid
Questions? Adverse drug event call:
(866) 562-2756
PHARBEST
NDC 16103-0377-11
Manufactured in the U.S.A.
†Compare to Tums® active ingredient
Calcium
Carbonate
Antacid
Calcium Rich •Low Sodium Antacid
THIS PACKAGE FOR HOUSEHOLDS
WITHOUT YOUNG CHILDREN
1000 TABLETS
10 gr (648 mg)
Pharbest Pharmaceuticals Inc.
