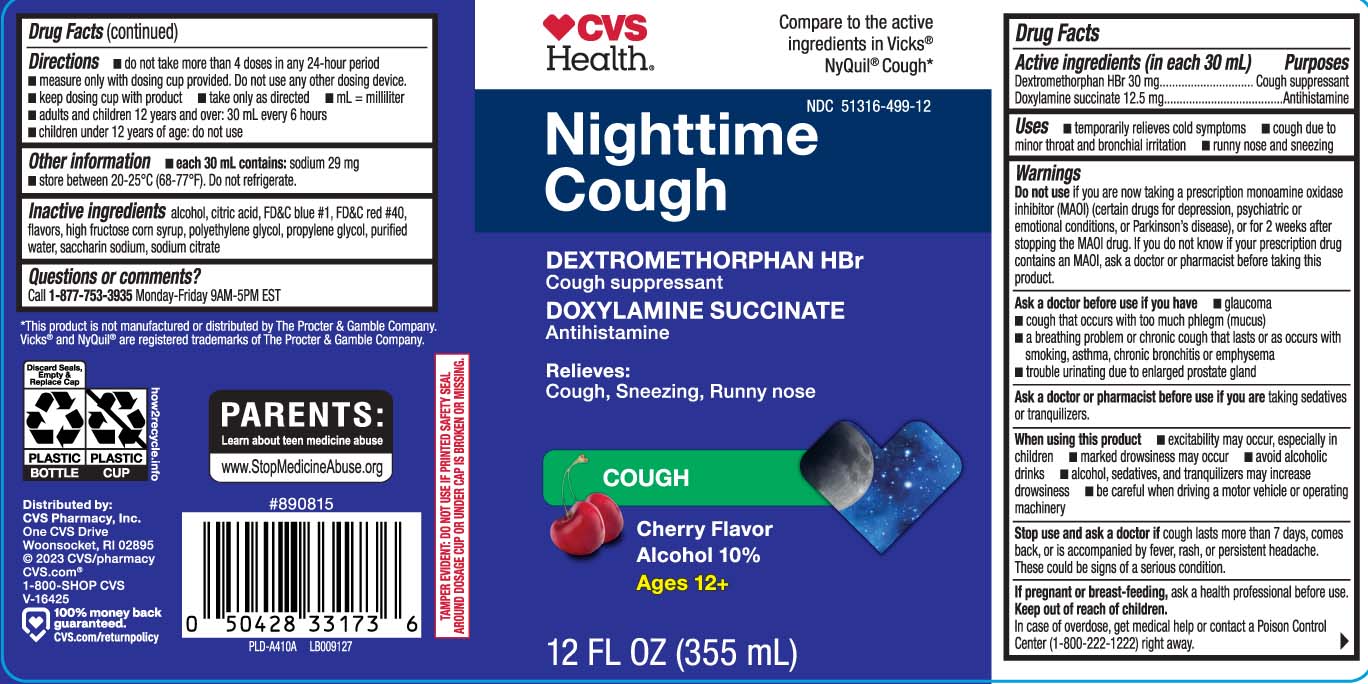
Uses
- temporarily relieves cold symptoms
- cough due to minor throat and bronchial irritation
- runny nose and sneezing
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- glaucoma
- cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis or emphysema
- trouble urinating due to enlarged prostate gland
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives, and tranquilizers may increase drowsiness
Directions
- do not take more than 4 doses in any 24-hours period
- measure only with dosing cup provided. Do not use any other dosing device
- keep dosing cup with product
- take only as directed
- mL = milliliter
- adults and children 12 years and over: 30 mL every 6 hours
- children under 12 years of age: do not use
Other information
- each 30 mL contains; sodium 29 mg
- store between 20-25ºC (68-77ºF). Do not refrigerate.
Inactive ingredients
alcohol, citric acid, FD&C blue 1, FD&C red 40, flavor, high fructose corn syrup, polyethylene glycol, propylene glycol, purified water, saccharin sodium, sodium citrate
Principal Display Panel
Compare to active ingredients in Vicks® NyQuil® Cough*
NightTime Cough
DEXTROMETHORPHAN HBr
Cough Suppressant
DOXYLAMINE SUCCINATE
Antihistamine
Relieves:
Cough, Sneezing, Runny nose
COUGH
Cherry Flavor
Alcohol 10%
Ages 12+
FL OZ (mL)
*This product is not manufactured or distributed by The Proctor & Gamble Company, Vicks® and NyQuil® are registered trademarks of The Procter & Gamble Company.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL AROUND DOSAGE CUP OR UNDER CAP IS BROKEN OR MISSING.
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive
Woocsocket, RI 02895