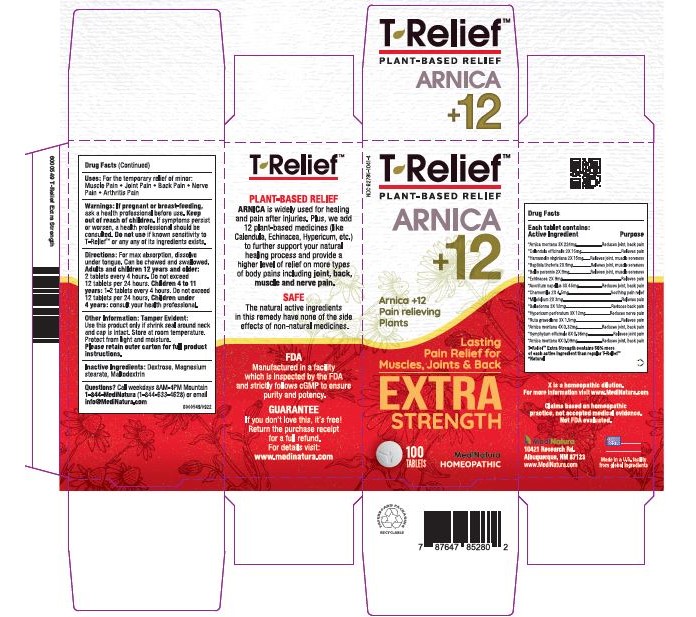
T-RELIEF EXTRA STRENGTH- arnica montana, calendula officinalis flowering, hamamelis virginiana root bark/stem, baptisia tinctoria root, bellis perennis, echinacea, unspecified, aconitum napellus, matricaria chamomilla, achillea millefolium, atropa belladonna, hypericum perforatum, ruta graveolens flowering top and comfrey root tablet, chewable
MediNatura Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
WARNINGS
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. If symptoms persist or worsen, a health professional should be consulted. Do not use if known sensitivity to T-Relief or any of its ingredients exists
USES
For the temporary relief of minor:
• Joint Pain
• Back Pain
• Muscular Pain
• Arthritis Pain
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
INDICATIONS
Relieves muscle, Joint, back, nerve and arthritis pain.
INGREDIENTS
Arnica montana 3X 62%, Calendula officinalis 2X 4%, Hamamelis virginiana 2X 4%, Baptisia tinctoria 2X 2.5%, Bellis perennis 2X 2.50%, Echinacea 2X 2.50%, Aconitum napellus 3X 12.5%, Chamomilla 2X 1.3%, Millefolium 2X 0.8%, Belladonna 3X 3.3%, Hypericum perforatum 3X 3%, Ruta graveolens 3X 0.4%, Arnica montana 4X 0.09%, Symphytum officinale 6X 0.1%, Arnica montana 8X 0.03%
INACTIVE INGREDIENT
Magnesium stearate,dextrose,maltodextrin.
Add image transcription here...
